
VAGIRUX 10 MICROGRAMOS COMPRIMIDOS VAGINALES


Cómo usar VAGIRUX 10 MICROGRAMOS COMPRIMIDOS VAGINALES
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para la usuaria
Vagirux 10 microgramos comprimidos vaginales
estradiol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Vagirux y para qué se utiliza
- Qué necesita saber antes de empezar a usar Vagirux
- Cómo usar Vagirux
- Posibles efectos adversos
- Conservación de Vagirux
Contenido del envase e información adicional
1. Qué es Vagirux y para qué se utiliza
Vagirux contiene estradiol.
- El estradiol es una hormona sexual femenina.
- Pertenece al grupo de hormonas llamadas estrógenos.
- Es exactamente igual al estradiol producido en los ovarios de las mujeres.
Vagirux pertenece al grupo de medicamentos llamados Terapia Hormonal Sustitutiva (THS) vaginal.
Vagirux se utiliza paraaliviar los síntomas menopáusicos de la vagina como sequedad o irritación. En términos médicos esto se conoce como “atrofia vaginal”. Está causado por un descenso de los niveles de estrógenos en su cuerpo. Esto ocurre de manera natural con la menopausia.
Vagirux actúasustituyendo el estrógeno que se produce normalmente en los ovarios de las mujeres. Se inserta en el interior de su vagina, para que la hormona sea liberada en el lugar donde se necesita. Esto puede aliviar las molestias en la vagina.
2. Qué necesita saber antes de empezar a usar Vagirux
Historia médica y revisiones regulares
El uso de THS conlleva riesgos que deben tenerse en cuenta al decidir si comenzar su uso o si se continúa el tratamiento.
La experiencia en el tratamiento de mujeres con menopausia prematura (debido a fallo o cirugía ovárica) es limitada. Si tiene una menopausia prematura, los riesgos de usar la THS pueden ser diferentes. Consulte a su médico.
Antes de empezar (o retomar) el uso de THS, su médico le preguntará acerca de su historial médico familiar. Su médico puede decidir llevar a cabo un examen físico. Esto puede incluir un examen de sus senos y/o examen interno, si es necesario.
Una vez haya empezado con Vagirux, debe consultar a su médico para que le haga revisiones al menos una vez al año. En estos chequeos, consulte a su médico sobre los beneficios y riesgos de continuar con Vagirux.
Acuda a realizarse exploraciones regulares de mama según le indique su médico.
No use Vagirux
Si se encuentra en alguna de las siguientes situaciones. Si no está segura sobre alguna de las siguientes condiciones, consulte a su médicoantes de usar Vagirux.
No use Vagirux:
- si padece o ha padecido cáncer de mama, o si sospecha que pueda tenerlo
- si padece o ha padecido cáncer dependiente de estrógenos como por ejemplo cáncer de las células que revisten el útero (endometrio) o si se sospecha que pueda tenerlo
- si sufre un sangrado vaginal anormal
- si sufre un crecimiento excesivo de las células que recubren el útero (hiperplasia de endometrio) que no está siendo tratado.
- si tiene o ha tenido coágulos sanguíneos en vena (trombosis) en las piernas (trombosis venosa profunda) o en los pulmones (embolismo pulmonar)
- si padece algún trastorno de la coagulación (como deficiencia de proteína C, proteína S o antitrombina).
- si padece o ha padecido previamente una enfermedad causada por coágulos de sangre en las arterias como un ataque al corazón, un accidente cerebrovascular o una angina de pecho
- si tiene o ha tenido una enfermedad del hígado y sus pruebas de función hepática no han vuelto a la normalidad
- si tiene un trastorno sanguíneo raro denominado “porfiria”, que se transmite de padres a hijos (hereditario)
- si es alérgica (hipersensible) al estradiol o a cualquiera de los demás componentes de Vagirux (incluidos en la sección 6 ” Contenido del envase e información adicional”)
Si alguna de las situaciones mencionadas anteriormente, aparecen por primera vez durante el uso de Vagirux, interrumpa el tratamiento y consulte a su médico inmediatamente.
Advertencias y precauciones
Consulte a su médico si sufre o ha sufrido alguno de los siguientes problemas antes de iniciar el tratamiento, ya que pueden retornar o empeorar durante el tratamiento con Vagirux. Si es así, consulte a su médico con mayor frecuencia para realizar exámenes médicos.
- Fibromas dentro del útero;
- Engrosamiento de las células que recubren el útero (endometriosis) o antecedentes de engrosamiento excesivo de las células que revisten el útero (hiperplasia endometrial);
- Riesgo elevado de desarrollar coágulos sanguíneos (ver “Coágulos sanguíneos en vena (trombosis)”;
- Riesgo elevado de contraer un cáncer dependiente de estrógenos (tales como, tener una madre, hermana o abuela que haya tenido cáncer de mama);
- Presión sanguínea elevada;
- Trastornos del hígado como tumores benignos del hígado;
- Diabetes;
- Piedras en la vesícula biliar (cálculos biliares);
- Migraña o dolores de cabeza graves;
- Una enfermedad del sistema inmune que afecta a muchos órganos del cuerpo (lupus eritematoso sistémico, LES);
- Epilepsia;
- Asma;
- Una enfermedad que afecta a la membrana del tímpano y el oído (otosclerosis);
- Un elevado nivel de grasa en sangre (triglicéridos);
- Retención de líquidos debido a problemas cardíacos o renales;
- Angioedema hereditario y adquirido.
Interrumpa el tratamiento con Vagirux y visite a su médico inmediatamente
Si usted experimenta alguna de las siguientes situaciones cuando utilice THS:
- cualquiera de las situaciones detalladas en la anterior sección “No use Vagirux”;
- color amarillento en piel o en lo blanco de los ojos (ictericia). Puede ser síntoma de enfermedad hepática;
- hinchazón de la cara, lengua o garganta y dificultad para tragar o urticaria acompañados de dificultad para respirar, que sugieren un angioedema;
- un gran aumento en su presión arterial (los síntomas pueden ser dolor de cabeza, cansancio y mareos);
- dolor de cabeza tipo migraña por primera vez;
- si se queda embarazada;
- si observa signos de un coágulo sanguíneo, tales como:
- inflamación con dolor y enrojecimiento de las piernas,
- dolor en el pecho repentino,
- dificultad para respirar.
Para más información ver “Coágulos sanguíneos en vena (trombosis)”
Nota:Vagirux no es un anticonceptivo. Si han transcurrido menos de 12 meses desde su último periodo menstrual o tiene menos de 50 años de edad, es posible que deba seguir usando métodos anticonceptivos adicionales para evitar el embarazo. Consulte con su médico para que le aconseje.
THS y cáncer
Engrosamiento excesivo del revestimiento del útero (hiperplasia de endometrio) y cáncer del revestimiento del útero (cáncer de endometrio)
El uso prolongado de comprimidos de THS con estrógenos solos puede aumentar el riesgo de desarrollar cáncer de revestimiento del útero (el endometrio).
No está claro si existe un riesgo similar con Vagirux cuando se usa para tratamientos repetidos o a largo plazo (más de un año). Sin embargo, Vagirux ha mostrado tener muy baja absorción en la sangre, y por lo tanto, la adición de un progestágeno no es necesaria.
No es preocupante si usted sufre sangrado o manchado, no obstante consulte a su médico. Podría ser una señal de engrosamiento del endometrio.
Los siguientes riesgos se aplican a los medicamentos deterapia hormonal sustitutiva(THS) que circulan en la sangre. Sin embargo, Vagirux es para el tratamiento local en la vagina, y la absorción en la sangre es muy baja. Es menos probable que las situaciones mencionadas a continuación empeoren o reaparezcan durante el tratamiento con Vagirux, pero debería consultar a su médico si está preocupada.
Cáncer de mama
La evidencia sugiere que usar Vagirux no aumenta el riesgo de cáncer de mama en mujeres que no hayan tenido cáncer de mama en el pasado. No se sabe si Vagirux puede usarse de forma segura en mujeres que hayan tenido cáncer de mama en el pasado.
Examine sus pechos regularmente. Acuda a su medico si detecta algún cambio como:
- surcos o hendiduras en la piel.
- cambios en el pezón.
- cualquier bulto que pueda ver o sentir.
Además, se aconseja participar en programas de exploraciones de las mamas cuando se lo ofrezcan.
Cáncer de ovario
El cáncer de ovario es raro, mucho más raro que el cáncer de mama. El uso de THS solo con estrógenos se ha asociado con un riesgo ligeramente mayor de cáncer de ovario. -
Comparación
El riesgo de cáncer de ovario varía con la edad. Por ejemplo, en mujeres de entre 50 y 54 años que no utilizan THS, alrededor de 2 mujeres por cada 2.000 serán diagnosticadas con cáncer de ovario en un período de 5 años. En mujeres que han estado utilizando THS durante 5 años, habrá aproximadamente 3 casos por cada 2.000 usuarias (es decir, alrededor de 1 caso adicional).
Efecto de THS en el corazón y la circulación
Coágulos sanguíneos en una vena (trombosis)
El riesgo de coágulos sanguíneos en las venas es aproximadamente de 1,3 a 3 veces superior en usuarias de THS en comparación con las no usuarias, especialmente durante el primer año de tratamiento.
Los coágulos sanguíneos pueden ser graves y si uno de ellos llega a los pulmones puede provocar dolor en el pecho, dificultad para respirar, desmayo o incluso la muerte.
Usted tiene mayor probabilidad de padecer coágulos sanguíneos a medida que se haga mayor y si alguno de los siguientes casos le afecta. Informe a su médico si presenta alguna de las siguientes situaciones:
- si no puede andar durante mucho tiempo a causa de una cirugía mayor, lesión o enfermedad (ver también sección 3, si debe someterse a cirugía)
- si padece un sobrepeso importante (IMC >30 kg/m2)
- si padece un problema de coagulación sanguínea que necesita un tratamiento prolongado con algún medicamento para prevenir los coágulos de sangre
- si algún familiar cercano ha padecido alguna vez un coágulo en piernas, pulmones u otros órganos
- si padece lupus eritematoso sistémico (LES)
- si tiene cáncer
En caso de indicios de coágulos sanguíneos ver “Interrumpa el tratamiento con Vagirux y visite a su médico inmediatamente”.
Comparación
En mujeres en la cincuentena que no están utilizando THS, se espera que una media de 4 a 7 de cada 1.000 tengan un coágulo de sangre en una vena en un periodo de 5 años.
En cuanto a mujeres en la cincuentena que han estado utilizando THS con estrógenos solos durante más de 5 años, será de 5 a 8 casos en 1.000 los usuarios (es decir, un caso adicional).
Enfermedad cardiaca (ataque al corazón)
En mujeres a que están utilizando terapia sólo con estrógenos no hay riesgo incrementado en padecer una enfermedad cardiaca.
Accidente cerebrovascular
El riesgo de accidente cerebrovascular es aproximadamente 1,5 veces superior en usuarias de THS en comparación con las no usuarias. El número de casos adicionales de accidente cerebrovascular debido al uso de THS aumenta con la edad.
Comparación
En mujeres en la cincuentena que no utilizan THS, un promedio de 8 de cada 1.000 sufrirán un accidente cerebrovascular durante un periodo medio de 5 años.
En mujeres en la cincuentena que utilizan THS, habrá 11 casos en 1.000 usuarias durante un periodo de 5 años (hasta 3 casos adicionales).
Otras condiciones
La THS no previene la pérdida de memoria. El riesgo de una probable pérdida de memoria puede ser algo superior en mujeres que empiezan a usar algún tipo de THS después de los 65 años. Consulte a su médico.
Uso de Vagirux con otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizando recientemente otros medicamentos, incluso los adquiridos sin receta, los elaborados a base de plantas medicinales u otros productos naturales. Sin embargo, Vagirux se utiliza para un tratamiento local en la vagina y no es probable que afecte a otros medicamentos. Vagirux puede afectar otros tratamientos aplicados por vía vaginal.
Fertilidad, Embarazo y lactancia
Vagirux es para uso en mujeres postmenopáusicas. Si se queda embarazada interrumpa el uso de Vagirux y contacte a su médico inmediatamente.
Conducción y uso de máquinas
No se conocen.
3. Cómo usar Vagirux
Siga exactamente las instrucciones de administración del medicamento indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
Uso del medicamento
- Puede empezar a usar Vagirux el día que mejor le convenga.
- Solo para uso vaginal. No tomar el comprimido por vía oral.
- Inserte el comprimido vaginal en su vagina con el aplicador.
En el apartado “INSTRUCCIONES DE USO” al final de este prospecto se le indica como hacerlo. Lea las instrucciones cuidadosamente antes de usar Vagirux.
El aplicador está diseñado para su uso múltiple, hasta 24 veces por una sola paciente (un comprimido vaginal por aplicación). Después, deseche el aplicador en la basura doméstica. No utilice aplicadores que muestren signos evidentes de deterioro.
Frecuencia de uso
- Aplicar un comprimido vaginal cada día durante las dos primeras semanas
- Posteriormente aplicar un comprimido vaginal dos veces a la semana. Entre cada una de las aplicaciones deberá transcurrir 3 ó 4 días.
Información general sobre el tratamiento de los síntomas de la menopausia
- Su médico tendrá que prescribirle la dosis más baja de Vagirux para tratar su síntoma por el tiempo que sea necesario. Consulte con su médico si cree que esta dosis es demasiado fuerte o no lo es.
- El tratamiento debería ser sólo continuado si el beneficio es mayor al riesgo. Consulte con su médico esta cuestión.
Si usa más Vagirux del que debe
- Si ha usado más Vagirux del que debiera, consulte con su médico o farmacéutico
- Vagirux se usa para el tratamiento local en el interior de la vagina. La dosis de estradiol es tan baja que se deberían usar un número considerable de comprimidos para conseguir la dosis normalmente usada para el tratamiento oral.
En caso de sobredosis o ingestión accidental, consulte con su médico o farmacéutico o llame al Servicio de Información Toxicológica. Teléfono 915 620 420, indicando el medicamento y la cantidad utilizada.
Si olvidó usar Vagirux
- Si olvidó una dosis, use el medicamento tan pronto como se acuerde
- No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Vagirux
No interrumpa el tratamiento con Vagirux sin consultar con su médico. Su médico le explicará los efectos de interrumpir el tratamiento. Él o ella tratarán con usted otras posibilidades de tratamiento.
Si debe someterse a cirugía
Si va a someterse a una intervención quirúrgica, informe al cirujano que está utilizando Vagirux. Puede que tenga que suspender el uso de Vagirux de 4 a 6 semanas antes de la operación para reducir el riesgo de coágulos sanguíneos (ver sección 2, "Coágulos sanguíneos en una vena”). Pregunte a su médico cuándo puede comenzar a utilizar Vagirux de nuevo.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas lo sufran.
Los siguientes trastornos han sido comunicados con más frecuencia en las mujeres que utilizan medicamentos de THS que circulan en sangre respecto a mujeres que no utilizan THS. Estos riesgos se presentan menos en los tratamientos administrados por vía vaginal como Vagirux:
- cáncer de ovario;
- coágulos sanguíneos en las venas de las piernas o los pulmones (tromboembolismo venoso);
- accidente vascular cerebral;
- probable pérdida de memoria si se empieza la THS con más de 65 años de edad;
Para más información sobre estos efectos secundarios, ver sección 2, “Qué necesita saber antes de empezar a usar Vagirux”.
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Dolor de cabeza
- Dolor de estómago
- Sangrado vaginal, flujo o molestias vaginales
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- Infección genital por hongos
- Mareo (náusea)
- Erupción cutánea
- Aumento de peso
- Sofocos
- Hipertensión
Muy raras: pueden afectar hasta 1 de cada 10.000 personas
- Diarrea
- Retención de líquidos
- Empeoramiento de la migraña
- Hipersensibilidad generalizada (p.ej. reacción o shock anafiláctico)
Los siguientes efectos adversos han sido reportados con el tratamiento sistémico de estrógenos:
- Enfermedad de la vesícula biliar
- Diferentes alteraciones de la piel
- decoloración de la piel especialmente en la cara o en el cuello, conocido como “manchas del embarazo” (cloasma)
- nódulos en la piel de color rojizo dolorosos (eritema nudoso)
- erupciones cutáneas con inflamaciones rojas o llagas (eritema multiforme)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Vagirux
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar el blister en el embalaje exterior para protegerlo de la luz.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Qué contiene Vagirux
- El principio activo es estradiol. Cada comprimido vaginal contiene estradiol hemihidrato equivalente a 10 microgramos de estradiol.
- Los demás componentes son: hipromelosa, lactosa monohidrato, almidón de maíz y estearato de magnesio.
- El recubrimiento contiene: hipromelosa y macrogol.
Aspecto del producto y contenido del envase
Los comprimidos vaginales son blancos, recubiertos, biconvexos, grabados con una E en una de sus caras. El diámetro del comprimido es de aproximadamente 6 mm.
Tamaño de los envases:
18 comprimidos vaginales con aplicador
24 comprimidos vaginales con aplicador
Puede que no todos los tamaños de envase estén comercializados.
Titular de la autorización de comercialización:
Gedeon Richter Plc.
Gyömroi út 19-21
1103 Budapest
Hungría
Responsable de la fabricación:
Gedeon Richter Plc.
Gyömroi út 19-21
1103 Budapest
Hungría
Haupt Pharma Münster GmbH (Member of the Aenova Group)
Schleebrüggenkamp 15
48159 Münster
Germany
Pueden solicitar más información respectoa este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Gedeon Richter Ibérica, S.A.
Sabino Arana, 28 - 4º 2ª
08028 Barcelona
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria: Rewellfem 10 Mikrogramm Vaginaltabletten
Croacia: Vagirux 10 mikrograma tablete za rodnicu
Dinamarca, Islandia, Liechtenstein: Rewellfem
Eslovaquia: Vagirux 10 mikrogramov vaginálne tablety
Eslovenia: VAGIRUX 10 mikrogramov vaginalne tablete
España: Vagirux 10 microgramos comprimidos vaginales
Estonia, Finlandia, Irlanda, Italia, Noruega, Polonia, Suecia, República Checa: Vagirux
Hungría: VAGIRUX 10 mikrogramm hüvelytabletta
Letonia: Vagirux 10 mikrogrami vaginalas tabletes
Lituania: VAGIRUX 10 mikrogramu makšties tabletes
Malta: Vagirux 10 microgram vaginal tablets
Portugal: Formyra
Rumanía: ESTRADIOL RICHTER 10 micrograme comprimate vaginale
Fecha de la última revisión de este prospecto:Abril 2022
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la {Agencia Española de Medicamentos y Productos Sanitarios (AEMPS): http://www.aemps.gob.es/
--------------------------------------------------------------------------------------------------------------------
INSTRUCCIONES DE USO
Cómo utilizar Vagirux
- Sacar el aplicador del blíster que lo contiene.
Abra por el final como se muestra en la imagen.
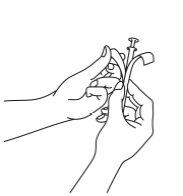
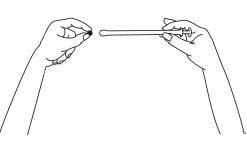
- Sostener el tubo y tirar del émbolo del aplicador hasta que se detenga. Colocar un comprimido vaginal en el soporte (extremo ancho) del tubo del aplicador.
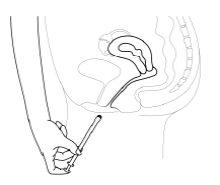
- El aplicador debe insertarse cuidadosamente en la vagina hasta el punto en que se siente cierta resistencia (8-10 cm).
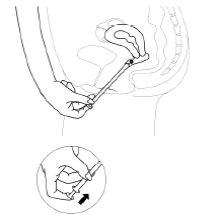
- Para liberar el comprimido vaginal, se debe presionar el émbolo hasta que se detenga. El comprimido quedará adherido a la pared vaginal de inmediato. No se caerá si la paciente se pone de pie o camina.
- Limpiar el aplicador después de cada uso, y antes de la siguiente aplicación de la siguiente manera:
- saque el émbolo del aplicador;
- limpiar tanto el tubo como el émbolo con jabón suave y enjuáguelos bien con agua tibia del grifo. Enjuague la parte interior y exterior del tubo.
- si es necesario, retire el agua restante tanto del tubo como del émbolo agitándolos levemente.
- secar el tubo y el émbolo al aire sobre una superficie limpia (p. ejem., una toalla de papel)
- reintroducir el émbolo y el tubo en el aplicador
- El aplicador deberá utilizarse hasta acabar el envase (18 ó 24 veces). Después, tirar a la basura doméstica.
- País de registro
- Precio medio en farmacia13.27 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VAGIRUX 10 MICROGRAMOS COMPRIMIDOS VAGINALESForma farmacéutica: GEL, 0,5 mgPrincipio activo: EstradiolFabricante: Orion CorporationRequiere recetaForma farmacéutica: GEL, 1 mgPrincipio activo: EstradiolFabricante: Orion CorporationRequiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 3 mgPrincipio activo: EstradiolFabricante: Merus Labs Luxco Ii S.À.R.L.Requiere receta
Médicos online para VAGIRUX 10 MICROGRAMOS COMPRIMIDOS VAGINALES
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VAGIRUX 10 MICROGRAMOS COMPRIMIDOS VAGINALES, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











