
BERTANEL 30 mg/1,5 ml SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar BERTANEL 30 mg/1,5 ml SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Bertanel y para qué se utiliza
- Qué necesita saber antes de empezar a usar Bertanel
- Cómo usar Bertanel
- Bertanel solo debe ser prescrito por médicos familiarizados con las diferentes características del medicamento y su modo de acción.
- Posibles efectos adversos
- Conservación de Bertanel
- Contenido del envase e información adicional
- BG
Introducción
Prospecto: información para el paciente
Bertanel30 mg/1,5 ml solución inyectable en jeringa precargada
metotrexato
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Bertanel y para qué se utiliza
- Qué necesita saber antes de empezar a usar Bertanel
- Cómo usar Bertanel
- Posibles efectos adversos
- Conservación de Bertanel
- Contenido del envase e información adicional
1. Qué es Bertanel y para qué se utiliza
Bertanel es un medicamento que tiene las siguientes propiedades:
- interfiere con el crecimiento de ciertas células del cuerpo que se multiplican rápidamente (agente antitumoral)
- atenúa las reacciones indeseadas del propio mecanismo de defensa del organismo (inmunosupresor) y
- tiene efectos antiinflamatorios
Bertanel se utiliza en pacientes con:
- artritis reumatoide activa (AR) en pacientes adultos.
- formas poliartríticas (cuando están implicadas cinco o más articulaciones) de artritis idiopática juvenil activa y grave (AIJ) cuando la respuesta a los antiinflamatorios no esteroideos (AINEs) ha sido inadecuada.
psoriasis recalcitrante discapacitante grave, que no responde adecuadamente a otras formas de tratamiento como fototerapia, PUVA y retinoides, y psoriasis grave que afecte a las articulaciones (artritis psoriásica) en pacientes adultos.
2. Qué necesita saber antes de empezar a usar Bertanel
Por favor pregunte a su médico o farmacéutico antes de usar Bertanel si tiene alguna duda. |
No use Bertanel:
- si es alérgico al metotrexato o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si tiene alguna enfermedad renal importante (su médico decide la gravedad de la enfermedad)
- si tiene alguna enfermedad hepática importante (su médico decide la gravedad de su enfermedad)
- si tiene trastornos del sistema que forma los componentes de la sangre
- si su consumo de alcohol es elevado
- si tiene el sistema inmunitario alterado
- si tiene una infección existente o grave, por ej., tuberculosis y VIH
- si tiene úlceras digestivas activas (incluyendo úlceras bucales)
- si está embarazada o en periodo de lactancia (ver sección “Embarazo, lactancia y fertilidad”)
- si se administran vacunas de virus vivos al mismo tiempo.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Bertanel si usted
- tiene diabetes mellitus tratada con insulina
- tiene infecciones crónicas inactivas (p. ej., tuberculosis, hepatitis B o C, herpes (herpes zoster))
- tiene o ha tenido alguna enfermedad del hígado o riñón
- tiene problemas con la función pulmonar
- tiene acumulación anormal de líquidos en el abdomen o en la cavidad situada entre los pulmones y la pared torácica (ascitis, derrame pleural)
- está deshidratado o padece alguna afección que pueda producir deshidratación (vómitos, diarrea, estomatitis).
Se ha notificado con metotrexato hemorragia pulmonar aguda en pacientes con enfermedad reumatológica subyacente. Si observa sangre al escupir o toser, se debe poner en contacto de forma inmediata con su médico.
El tratamiento se administra una vez a la semana.
La administración incorrecta de metotrexato puede producir efectos secundarios graves, incluyendo potencialmente letales.
Lea la sección 3 de este prospecto con detenimiento.
El metotrexato puede hacer que la piel sea más sensible a la luz solar. Evite el sol intenso y no utilice camas de bronceado ni lámparas ultravioletas sin consejo médico. Para proteger la piel del sol intenso, lleve ropa adecuada o utilice un protector solar con un factor de protección alto.
Si ha tenido problemas de piel después de una radioterapia (dermatitis inducida por radiación) y quemaduras solares, estas afecciones pueden reaparecer durante el tratamiento con metotrexato (reacción de memoria).
Si usted, su pareja o su cuidador notan la aparición o un empeoramiento de síntomas neurológicos, como debilidad muscular general, alteraciones de la visión, cambios en el pensamiento, la memoria y la orientación que generan confusión y cambios en la personalidad, contacte con su médico inmediatamente porque estos pueden ser síntomas de una infección cerebral grave muy rara denominada leucoencefalopatía multifocal progresiva (LMP).
Niños y adolescentes
Las instrucciones de administración dependen del peso corporal del paciente. No se recomienda el uso en niños menores de 3 años debido a que la experiencia con este grupo de edad es insuficiente.
Los niños en tratamiento con Bertanel deben seguir un control particularmente frecuente por el médico especialista en este área, para identificar los posibles efectos adversos lo más pronto posible.
Pacientes de edad avanzada
Los pacientes de edad avanzada en tratamiento con metotrexato deben estar bajo vigilancia médica estrecha por un especialista, para que se puedan detectar posibles efectos adversos lo antes posible.
El deterioro de las funciones de hígado y riñón, asociados a la edad, así como tener bajas las reservas de ácido fólico en edad avanzada, requieren la administración de una dosis relativamente baja de metotrexato.
Las alteraciones cutáneas producidas por psoriasis pueden empeorar durante el tratamiento con Bertanel si al mismo tiempo se expone a radiación UV.
Exploraciones recomendadas y precauciones:
Incluso cuando Bertanel se administre a dosis bajas, se pueden producir efectos adversos graves. Para detectarlos lo antes posible, su médico debe realizarle revisiones de seguimiento y pruebas de laboratorio.
Antes de iniciar el tratamiento:
Antes de iniciar el tratamiento, se le hará un análisis de sangre para comprobar si usted tiene suficientes células sanguíneas. También se comprobará en su sangre su función hepática y si tiene hepatitis. Además se comprobará la albúmina sérica (una proteína de la sangre), el estado de hepatitis (infección hepática) y la función renal. Puede que su médico decida realizar también otras pruebas hepáticas, algunas podrían ser imágenes de su hígado y otras podrían necesitar una pequeña muestra de tejido de su hígado, para examinarlo más atentamente. Puede que su médico también compruebe si usted tiene tuberculosis y se le podría hacer una radiografía de tórax o una prueba de función pulmonar.
Durante el tratamiento:
Su médico puede llevar a cabo los siguientes exámenes:
-examen de la cavidad oral y de la faringe para detectar cambios en la membrana mucosa, como inflamación o úlceras
-análisis de sangre/recuento sanguíneo, con número de células sanguíneas y medición de los niveles séricos de metotrexato
-análisis de sangre para controlar la función del hígado
-pruebas de imagen para controlar la función del hígado
-tomar una pequeña muestra de tejido del hígado para examinarlo más atentamente
-análisis de sangre para controlar la función del riñón
-control del tracto respiratorio y, si es necesario, prueba de la función pulmonar
Es muy importante que usted acuda a hacerse estas pruebas programadas.
Si los resultados de alguna de estas pruebas son llamativos, su médico le ajustará el tratamiento como corresponda.
Medidas de precaución especiales para el tratamiento con Bertanel
El metotrexato afecta de manera temporal a la producción de espermatozoides y óvulos, lo que es reversible en la mayoría de los casos. El metotrexato puede causar abortos y defectos de nacimiento graves. Si es usted mujer, debe evitar quedarse embarazada mientras utilice metotrexato y durante al menos seis meses después de haber finalizado el tratamiento. Si es usted hombre, debe evitar engendrar un hijo si se le está administrando metotrexato en ese momento y durante al menos 3 meses después de finalizar su tratamiento. Ver también sección «Embarazo, lactancia y fertilidad».
Uso de Bertanel con otros medicamentos
Comunique a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento. Recuerde informar a su médico sobre su tratamiento con Bertanel si le recetan otro medicamento cuando todavía está en tratamiento.
Es especialmente importante que informe a su médico si está usando:
- otros tratamientos para la artritis reumatoide o la psoriasis, tales como leflunomida, sulfasalazina (también utilizada para la colitis ulcerosa), aspirina, fenilbutazona o amidopirina
- alcohol (debe evitarse)
- vacunas de virus vivos
- azatioprina (utilizada para prevenir el rechazo después de un trasplante de órgano)
- retinoides (utilizados para tratar psoriasis y otras enfermedades de la piel)
- medicamentos anticonvulsivantes (previenen las crisis epilépticas)
- tratamientos contra el cáncer
- barbitúricos (ayudan a dormir)
- tranquilizantes
- anticonceptivos orales
- probenecid (para la gota)
- antibióticos
- Las penicilinas pueden reducir la excreción de metotrexato causando un aumento potencial de los efectos adversos
- metamizol (sinónimos novaminsulfon y dipirona) (medicamento para el dolor intenso y/o la fiebre)
- pirimetamina (utilizada para prevenir y tratar la malaria)
- preparados vitamínicos que contienen ácido fólico
- inhibidores de la bomba de protones (utilizados para tratar la acidez de estómago o las úlceras)
- teofilina (utilizada para tratar el asma)
Uso de Bertanel con alimentos, bebidas y alcohol
Durante el tratamiento con Bertanel no debe consumir alcohol y debe evitar el consumo excesivo de café, refrescos que contengan cafeína y té negro.
Debe asegurarse la ingestión de gran cantidad de líquidos durante el tratamiento con Bertanel, ya que la deshidratación (reducción del agua del organismo) puede aumentar la toxicidad de Bertanel.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
No utilice Bertanel durante el embarazo o si está intentando quedarse embarazada. El metotrexato puede causar defectos de nacimiento, dañar al feto o provocar abortos. Se asocia a malformaciones del cráneo, la cara, el corazón y los vasos sanguíneos, el cerebro y las extremidades. Por ello, es muy importante que no se administre metotrexato a pacientes embarazadas o que tengan previsto quedarse embarazadas. En mujeres en edad fértil se debe excluir cualquier posibilidad de embarazo con las medidas oportunas, por ejemplo, una prueba de embarazo antes de empezar el tratamiento.
Debe evitar quedarse embarazada mientras toma metotrexato y durante al menos 6 meses después de suspender el tratamiento, utilizando para ello métodos anticonceptivos fiables durante todo este tiempo (ver también sección ”Advertencias y precauciones”).
Si se queda embarazada durante el tratamiento o sospecha que podría estar embarazada, consulte a su médico lo antes posible. Se le debe ofrecer información sobre el riesgo de efectos perjudiciales para el niño durante el tratamiento.
Si desea quedarse embarazada, consulte a su médico, quien puede derivarle a un especialista para que le informe antes del comienzo previsto del tratamiento.
Lactancia
No debe amamantar al niño durante el tratamiento, ya que el metotrexato pasa a la leche materna. Si su médico considera que el tratamiento con metotrexato es absolutamente necesario, se debe interrumpir la lactancia materna.
Fertilidad del varón
Los datos disponibles no indican un mayor riesgo de malformaciones ni abortos si el padre toma una dosis de metotrexato inferior a 30 mg/semana. Sin embargo, no se puede descartar por completo este riesgo. El metotrexato puede ser genotóxico, lo que significa que puede causar mutaciones genéticas. El metotrexato puede afectar a la producción de espermatozoides y causar defectos de nacimiento. Por tanto, debe evitar engendrar un hijo o donar semen mientras toma metotrexato y durante al menos 3 meses después de interrumpir el tratamiento.
Conducción y uso de máquinas
Durante el tratamiento con Bertanel pueden producirse efectos adversos que afectan al sistema nervioso central como fatiga y mareos. En algunos casos la capacidad para conducir vehículos y/o utilizar máquinas puede verse afectada. Si se siente cansado o mareado, no conduzca ni maneje herramientas o máquinas.
Bertanel contiene cloruro sódico e hidróxido sódico
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis semanal; esto es esencialmente “exento de sodio”.
3. Cómo usar Bertanel
Advertencia importante sobre la dosis de Bertanel (metotrexato):
Utilice Bertanel solo una vez por semanapara el tratamiento de artritis reumatoide, formas poliartríticas de artritis idiopática juvenil o psoriasis. El uso excesivo de Bertanel (metotrexato) puede ser mortal. Lea la sección 3 de este prospecto con mucha atención. Si tiene alguna duda, consulte a su médico o farmacéutico antes de tomar este medicamento.
Bertanel solo debe ser prescrito por médicos familiarizados con las diferentes características del medicamento y su modo de acción.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Bertanel se administra sólouna vez por semana. Junto con su médico, usted decidirá un día adecuado cada semana para recibir su inyección.
La administración incorrecta de Bertanel puede producir efectos secundarios graves, incluyendo potencialmente letales.
La dosis normal es:
Posología en pacientes con artritis reumatoide
La dosis inicial recomendada es de 7,5 mg de metotrexato una vez por semana. Bertanel se administra en una sola aplicación en forma de inyección bajo la piel, en un músculo o en una vena (ver sección “Método y duración de la administración”).
Si esto no es suficiente y si tolera bien el medicamento, las dosis de Bertanel pueden aumentarse. La dosis media semanal es de 15-20 mg. Por lo general, no se debe superar una dosis semanal de 25 mg de Bertanel. Una vez alcanzado el resultado terapéutico deseado, si es posible, la dosis debe ser reducida gradualmente hasta la dosis eficaz de mantenimiento más baja posible.
Posología en niños y adolescentes menores de 16 años de edad con formas poliartríticas de artritis idiopática juvenil
La dosis recomendada es de 10-15 mg/m2 de superficie corporal por semana. En casos de respuesta inadecuada, la dosis semanal puede aumentarse hasta 20 mg/m2 de superficie corporal por semana. Sin embargo, se deben realizar controles periódicos más a menudo. Como hay muy pocos datos sobre la administración intravenosa (en vena) en niños y adolescentes, solo se debe administrar mediante inyección subcutánea (bajo la piel) o intramuscular (en el músculo).
No se recomienda el uso en niños menores de 3 años, ya que la experiencia en este grupo de edad es insuficiente.
Adultos con formas graves de psoriasis vulgar o artritis psoriásica
Se recomienda administrar una dosis de prueba de 5-10 mg para evaluar los posibles efectos nocivos.
Esta dosis se puede administrar por vía subcutánea (bajo la piel), intramuscular (en el músculo) o intravenosa (en vena).
Si, una semana más tarde, no se han observado cambios en el recuento sanguíneo, el tratamiento continuará con una dosis de aproximadamente 7,5 mg. La dosis se puede aumentar de forma progresiva (en etapas de 5-7,5 mg por semana y controlando el recuento sanguíneo) hasta obtener los resultados terapéuticos deseados. Generalmente, una dosis semanal de 20 mg puede asociarse con aumentos significativos en la toxicidad. No se debe sobrepasar una dosis semanal de 30 mg.
Una vez alcanzado el resultado terapéutico deseado, la dosis se deberá reducir semanalmente hasta alcanzar la dosis eficaz de mantenimiento más baja posible para el paciente.
Pacientes con insuficiencia renal
Los pacientes con insuficiencia renal pueden necesitar dosis menores.
Método y duración de la administración
El médico determinará la duración del tratamiento. ¡Bertanel se inyecta una vez por semana!Se recomienda especificar un día determinado de la semana como “día de la inyección”.
Bertanel se administra en forma de inyección bajo la piel, en el músculo o en vena; en niños y adolescentes no se debe administrar por vía intravenosa.
El tratamiento con Bertanel de la artritis reumatoide, artritis idiopática juvenil, psoriasis vulgar y artritis psoriásica es un tratamiento de larga duración.
Artritis reumatoide
Generalmente se puede esperar una mejoría de los síntomas al cabo de 4-8 semanas de tratamiento. Los síntomas pueden volver a aparecer tras la interrupción del tratamiento con Bertanel.
Formas graves de psoriasis vulgar y artritis psoriásica (psoriasis artropática)
Generalmente, la respuesta al tratamiento se puede esperar al cabo de 2-6 semanas.
Dependiendo del cuadro clínico y de los cambios en los parámetros de laboratorio, el tratamiento se continuará o suspenderá.
Al comienzo del tratamiento, Bertanel podrá ser inyectado por el personal médico. Sin embargo, es posible que su médico decida que usted puede aprender a inyectarse Bertanel usted mismo. Recibirá la formación adecuada para ello. En ninguna circunstancia debe intentar inyectarse usted mismo, a menos que se le haya enseñado a hacerlo.
Si usa más Bertanel del que debe
Siga las recomendaciones sobre las dosis dadas por su médico. No cambie la dosis por su cuenta.
Si sospecha que usted (o alguien más) se ha administrado demasiado Bertanel, por favor contacte con su médico o acuda inmediatamente al hospital más cercano o consulte al Servicio de Información Toxicológica, teléfono 91 562 04 20 indicando el medicamento y la cantidad usada. Él/ella decidirán sobre las medidas de tratamiento necesarias, dependiendo de la gravedad de la intoxicación.
Una sobredosis con metotrexato puede producir reacciones tóxicas graves. Los síntomas de sobredosis pueden incluir hemorragias o hematomas, debilidad inusual, llagas en la boca, náuseas, vómitos, heces negras o con sangre, tos con sangre o vómito parecido a los posos de café, y disminución de la producción de orina. Ver sección 4.
Lleve el envase del medicamento consigo si acude al médico o a un hospital.
El antídoto en caso de sobredosis es el folinato cálcico.
Si olvidó usar Bertanel
No use una dosis doble para compensar las dosis olvidadas, continúe utilizando la dosis recetada. Si tiene dudas, consulte a su médico.
Si interrumpe el tratamiento con Bertanel
No debe interrumpir el tratamiento con Bertanel a menos que se lo haya indicado su médico. Si sospecha efectos adversos graves, consulte inmediatamente con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe inmediatamente a su médico si presenta sibilancias, dificultad para respirar, hinchazón de los párpados, cara o labios, erupción o picor (especialmente si afecta a todo el cuerpo) y si siente que va a desmayarse (estos podrían ser síntomas de reacciones alérgicas graves o shock anafiláctico).
Efectos adversos graves
Si aparece alguno de los siguientes efectos adversos, contacte inmediatamente con su médico:
- molestias pulmonares (los síntomas pueden ser malestar general, tos irritativa y seca, dificultad para respirar, sensación de falta de aire en reposo, dolor en el pecho o fiebre)
- sangre al escupir o toser
- formación de ampollas o descamación grave de la piel (esto también puede afectar a la boca, ojos y genitales)
- hemorragias no habituales (incluidos vómitos con sangre) o hematomas
- diarrea grave
- úlceras en la boca
- heces negras o como la brea
- sangre en orina o en heces
- pequeñas manchas rojas en la piel
- fiebre
- coloración amarilla de la piel (ictericia)
- dolor o dificultad para orinar
- hinchazón de manos, tobillos o pies o cambios en la frecuencia de micción o disminución o ausencia de orina (síntomas de fallo renal)
- sed y/o necesidad de orinar con frecuencia
- ataques (convulsiones)
- pérdida de conciencia
- visión borrosa o disminución de la visión
También se han comunicado los siguientes efectos adversos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- pérdida del apetito, náuseas (sintiendo mareos), vómitos, dolor de tripa
- inflamación y úlceras en la boca y la garganta
- aumento de las enzimas hepáticas.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- formación reducida de células sanguíneas con disminución de leucocitos y/o glóbulos rojos y/o plaquetas (leucopenia, anemia, trombocitopenia)
- dolor de cabeza
- cansancio, somnolencia
- hormigueo, cosquilleo, pinchazos o ardor de la piel, urticaria, enrojecimiento de la piel, picor
- inflamación de los pulmones (neumonitis)
- diarrea.
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- herpes (herpes zoster)
- linfoma (que en algunos casos remitió espontáneamente tan pronto como se interrumpió el tratamiento con Bertanel)
- disminución del número de células sanguíneas y plaquetas
- reacciones alérgicas graves
- diabetes
- depresión
- debilidad de todo el lado izquierdo o derecho del cuerpo
- mareo, confusión
- convulsiones
- daño cerebral (leucoencefalopatía/encefalopatía)
- inflamación de los vasos sanguíneos
- daño pulmonar, edema pulmonar
- úlceras y hemorragias en el tubo digestivo
- inflamación del páncreas
- trastornos hepáticos
- disminución de proteínas en sangre
- urticaria (sola), sensibilidad a la luz, coloración marrón de la piel
- reacciones tóxicas graves de la piel incluyendo formación de ampollas y desprendimiento de la capa superior de la piel (síndrome de Stevens-Johnson, síndrome de Lyell)
- caída del pelo
- incremento de los nódulos reumáticos
- psoriasis dolorosa
- reacciones similares a quemaduras solares debido a una mayor sensibilidad de la piel a la luz solar
- dolor muscular o de las articulaciones
- osteoporosis (reducción de la masa ósea)
- inflamación y úlceras de la vejiga (posiblemente con presencia de sangre en la orina), dolor al orinar
- malformaciones fetales
- inflamación y úlceras en la vagina
- sensación de ardor o daño del tejido tras la inyección de Bertanel en el músculo.
Raros (pueden afectar hasta 1 de cada 1.000 personas):
- sepsis
- glóbulos rojos muy grandes (anemia megaloblástica)
- alteraciones del estado de ánimo
- problemas temporales con la percepción
- debilidad del movimiento voluntario en todo el cuerpo
- problemas con el habla
- graves problemas oculares
- tensión arterial baja
- coágulos de sangre
- dolor de garganta
- interrupción de la respiración
- inflamación del tubo digestivo, heces con sangre
- inflamación de las encías
- hepatitis aguda (inflamación del hígado)
- cambios de color en las uñas, caída de uñas
- acné, manchas rojas o púrpura por roturas de vasos sanguíneos
- fracturas de huesos debido al esfuerzo
- alteraciones electrolíticas
- aborto
- formación defectuosa del esperma
- alteraciones de la menstruación.
Muy raros (pueden afectar hasta 1 de cada 10.000 personas):
- herpes labial (herpes simple)
- hepatitis
- insuficiencia grave de médula ósea
- inmunodeficiencia (hipogammaglobulinemia)
- dolor
- debilidad muscular
- alteraciones del gusto (sabor metálico)
- inflamación del revestimiento del cerebro que produce parálisis o vómitos
- ojos rojos
- inflamación de la cavidad que rodea el corazón, líquido en la cavidad que rodea el corazón
- neumonía, problemas respiratorios, asma
- vómitos con sangre
- fallo hepático
- infección alrededor de las uñas, forúnculos, pequeños vasos sanguíneos en la piel
- proteínas en la orina
- muerte fetal
- problemas en la formación de óvulos (mujer) y esperma (hombre)
- pérdida del deseo sexual
- problemas de erección
- flujo vaginal
- infertilidad
- leves reacciones locales en la piel si Bertanel se administra por debajo de la piel
- trastornos linfoproliferativos (aumento excesivo de glóbulos blancos)
- sensación de entumecimiento u hormigueo/sensibilidad a los estímulos menor de la normal
Frecuencia no conocida (no pueden estimarse a partir de los datos disponibles):
- infecciones que en algunos casos pueden ser mortales
- inflamación de los nódulos linfáticos
- mal funcionamiento del sistema inmunológico
- fiebre
- inflamación de pequeños vasos sanguíneos causada por una reacción alérgica
- inflamación del revestimiento del abdomen
- lenta cicatrización de las heridas
- hemorragia pulmonar
- lesión en los huesos de la mandíbula (secundaria a un aumento excesivo de glóbulos blancos)
- destrucción del tejido en el lugar de la inyección
- enrojecimiento y descamación de la piel
- hinchazón
Cuando se administra metotrexato por vía intramuscular, se pueden producir frecuentemente en el lugar de inyección reacciones adversas locales (sensación de quemazón) o lesiones (formación de abscesos estériles, destrucción del tejido graso). La administración de metotrexato por vía subcutánea posee una buena tolerancia local. Sólo se han observado reacciones locales leves en la piel, disminuyendo durante el tratamiento.
Metotrexato puede reducir el número de leucocitos, pudiendo disminuir su resistencia a la infección. Si sufre una infección con síntomas como fiebre y deterioro grave de su estado general, o fiebre con síntomas de infección local como dolor de garganta/faringitis/dolor en la boca o problemas urinarios, debe acudir a su médico inmediatamente. Se realizará un análisis de sangre para comprobar una posible disminución de leucocitos (agranulocitosis). Es importante informar a su médico sobre su medicamento.
Metotrexato puede producir efectos adversos graves (a veces peligrosos para la vida). Por tanto, su médico le realizará pruebas para comprobar las anomalías provocadas en la sangre (por ej. disminución de leucocitos, disminución de plaquetas, linfoma) y cambios en el riñón y en el hígado.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Bertanel
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la jeringa precargada y en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en el envase original para protegerlo de la luz.
No conservar a temperatura superior a 25ºC.
El producto debe utilizarse inmediatamente después de abrir el envase.
No debe utilizar Bertanel si la solución no es transparente y contiene partículas.
Para un solo uso. ¡Desechar cualquier resto de solución sin utilizar!
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Bertanel
El principio activo es metotrexato.
1 ml de solución inyectable contiene 20 mg de metotrexato (equivalente a 21,94 mg de metotrexato disódico).
1 jeringa precargada de 1,5 ml de solución inyectable contiene 30 mg de metotrexato.
Los demás componentes son: cloruro sódico, hidróxido sódico para ajuste de pH y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Bertanel es una solución inyectable que se comercializa en jeringas precargadas con una solución para inyección transparente amarillenta.
Cada caja contiene 1 jeringa precargada o envases multipack conteniendo 4, 5, 6, 12 ó 30 jeringas precargadas con 1,5 ml de solución inyectable, agujas de inyección de un solo uso con o sin cánula de seguridad y algodones empapados en alcohol.
Puede que solamente se comercialicen algunos tamaños de envase.
Titular de la autorización de comercialización
Ebewe Pharma Ges.m.b.H. Nfg.KG
Mondseestrasse 11
4866 Unterach, Austria
Responsable de la fabricación
Ebewe Pharma Ges.m.b.H. Nfg.KG
Mondseestrasse 11
4866 Unterach, Austria
Salutas Pharma GmbH
Otto-von-Guericke-Alle 1
D-39179 Barleben
Alemania
Fareva Unterach GmbH
Mondseestraße 11
4866 Unterach
Austria
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Representante local
Laboratorios Farmacéuticos ROVI, S.A.
Julián Camarillo, 35
28037 Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Nombre del estado miembro | Nombre del medicamento | Titular |
AT | Ebetrexat 20mg/ml Injektionslösung in einer Fertigspritze | EBEWE Pharma Ges.m.b.H. Nfg. KG |
BE | Ebetrexat 20mg/ml, solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
BG | Ebetrexat 20mg/ml, solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
CZ | Methotrexat Ebewe 20mg/ml, roztok na injekce, predplnená injekcni strikacka | EBEWE Pharma Ges.m.b.H. Nfg. KG |
DE | MTX Sandoz 20mg/ml Injektionslösung, in einer Fertigspritze | Sandoz Pharmaceuticals GmbH |
DK | Ebetrex | EBEWE Pharma Ges.m.b.H. Nfg. KG |
ES | Bertanel 30 mg/1,5 ml solución inyectable en jeringa precargada. | EBEWE Pharma Ges.m.b.H. Nfg. KG |
EE | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
FI | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
HU | Ebetrexat 20mg/ml, oldatos injekcio eloretöltött fecskendoben | EBEWE Pharma Ges.m.b.H. Nfg. KG |
IT | TREXODEM 20mg/ml-soluzione iniettabile, siringhe preriempite | Sandoz S.p.A. |
LT | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
LU | Methotrexat Sandoz 20mg/ml, solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
LV | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
NL | Ebetrex 20 mg = ml, oplossnig voor injectie in voorgevulde injectiespuit 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
NO | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
PL | Ebetrexat | EBEWE Pharma Ges.m.b.H. Nfg. KG |
PT | Methotrexato Sandoz, solução injectável, seringa pré-cheia | EBEWE Pharma Ges.m.b.H. Nfg. KG |
RO | Methrotrexate Ebewe 20 mg/ml solutie injectabila în seringa preumpluta | EBEWE Pharma Ges.m.b.H. Nfg. KG |
SE | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
SK | Methotrexat Ebewe 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
SI | Metotreksat “Ebewe” 20 mg/ml raztopina za injiciranje, napolnjena injekcijska brizga | EBEWE Pharma Ges.m.b.H. Nfg. KG |
UK | Ebetrex 20mg/ml solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
Fecha de la última revisión de este prospecto:septiembre 2024
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
-----------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Bertanel 30 mg/1,5 ml solución inyectable en jeringa precargada
Instrucciones de uso, manipulación y eliminación
La solución debe ser transparente y sin partículas.
La manipulación y la eliminación del medicamento deben realizarse igual que con los demás preparados citotóxicos y de acuerdo con la normativa local. Si alguna mujer del personal sanitario estuviera embarazada, no deberá manipular y/o administrar Bertanel.
Para un solo uso. Desechar cualquier resto de solución sin utilizar.
La eliminación del medicamento no utilizado o de los materiales de desecho, se realizará de acuerdo con la normativa local.
Incompatibilidades
En ausencia de estudios de compatibilidad, este medicamento no debe mezclarse con otros.
Precauciones especiales de conservación
Conservar en el envase original para protegerlo de la luz.
No conservar a temperatura superior a25ºC.
Instrucciones paso a paso para la inyección subcutánea:
Paso 1:
- Saque de la caja el envase interior que contiene la jeringa precargada, la cánula y la aguja.
- Abra el envase interior tirando de la solapa de la esquina. Saque la jeringa precargada.
- Gire el tapón de goma gris recubierto con plástico de la jeringa, sin tocar la abertura de la jeringa precargada (véase la figura 1).
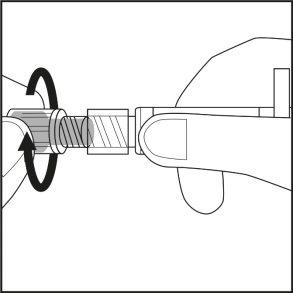
Figura 1.
Paso 2:
- Coloque la jeringa de nuevo en el envase interior. La solución de color amarillo no se derramará.
- Compruebe la etiqueta del estuche de plástico que contiene la aguja. La etiqueta debe estar intacta (véase la figura 2).
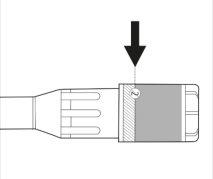
Figura 2.
Paso 3:
- Retire la tapa del estuche de plástico de la aguja girándola y tirando a continuación. Véase la figura 3.1
- Gire cuidadosamente la aguja junto con el estuche de plástico en la jeringa hasta donde llegue. Véase la figura 3.2
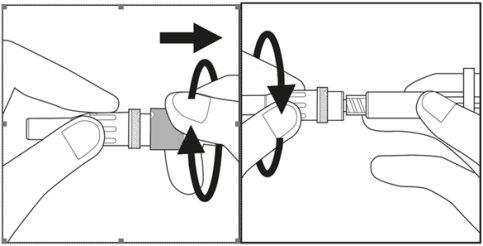
Figura 3.1 Figura 3.2
Paso 4:
- Elija el punto de inyección en la zona del vientre o de los muslos y límpielo con algodón empapado en alcohol. No toque esta zona antes de la inyección (véase las figuras 4.1 y 4.2).
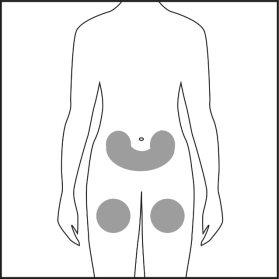
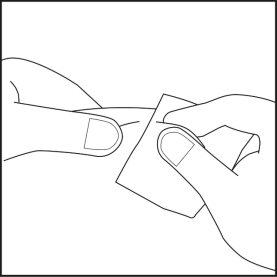
Figura 4.1 Figura 4.2
Paso 5:
- Retire la funda de la cánula y déjela aparte.
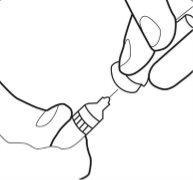
- No toque la cánula estéril. Si esto llegara a ocurrir, pregunte a su médico o farmacéutico sobre la posibilidad de utilizar otra cánula. Con dos dedos, forme un pliegue en la piel y, a continuación, introduzca la aguja en él casi verticalmente.
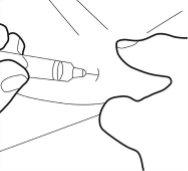
Paso 6:
- Introduzca la cánula completamente en el pliegue de la piel. Luego, lentamente, empuje el émbolo hacia abajo e inyecte todo el líquido bajo la piel.
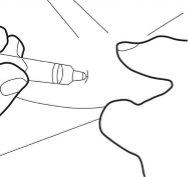
Paso 7:
- Retire con cuidado la cánula y empape el lugar de la inyección con un algodón humedecido en alcohol. No frote pues puede producirse irritación en el lugar de la inyección.
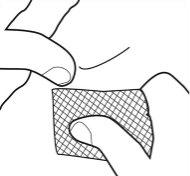
- Para evitar lesiones, deseche las jeringas utilizadas en un contenedor.
- País de registro
- Precio medio en farmacia29.38 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BERTANEL 30 mg/1,5 ml SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 10 mg/ 1 mlPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 15 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere recetaForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: MetotrexatoFabricante: Ebewe Pharma Ges.M.B.H. Nfg.KgRequiere receta
Médicos online para BERTANEL 30 mg/1,5 ml SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BERTANEL 30 mg/1,5 ml SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






