
NICORETTE SUPERMINT 2 mg LOZENGES


How to use NICORETTE SUPERMINT 2 mg LOZENGES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Nicorette Supermint 2 mg lozenges
Nicotine
Read the entire leaflet carefully before starting to take this medication because it contains important information for you.
Follow the administration instructions of the medication contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
- You should consult a doctor if after 6 months of treatment you still have difficulties quitting smoking without the help of Nicorette Supermint.
Contents of the package leaflet:
- What is Nicorette Supermint and what is it used for
- What you need to know before taking Nicorette Supermint
- How to take Nicorette Supermint
- Possible side effects
- Storage of Nicorette Supermint
- Package contents and additional information
1. What is Nicorette Supermint and what is it used for
This medication is used to alleviate withdrawal symptoms and reduce nicotine cravings that appear when trying to quit smoking. This treatment is indicated for adult smokers aged 18 and over.
Nicorette Supermint 2 mg lozenges are suitable for smokers with low nicotine dependence, such as those who smoke their first cigarette of the day at least 30 minutes after waking up or those who smoke 20 cigarettes or less per day.
Nicorette Supermint alleviates nicotine withdrawal symptoms, including anxiety, associated with tobacco cessation. When you suddenly stop providing nicotine to your body, you are affected by various unpleasant sensations, known as withdrawal symptoms, such as irritability or aggression, depression, anxiety, restlessness, concentration problems, increased appetite or weight gain, craving to smoke (anxiety), nighttime awakenings or sleep disorders. The nicotine in Nicorette Supermint can help prevent or reduce these unpleasant symptoms, as well as your desire to smoke.
To improve the chances of quitting smoking, it is recommended to seek medical advice and support.
2. What you need to know before taking Nicorette Supermint
Do not take Nicorette Supermint
- If you are allergicto nicotine or any of the other components of this medication (listed in section 6).
- If you are under 18 years old.
- If you are a non-smoker.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Nicorette Supermint.
Inform your doctor before taking this medication if you suffer from any of the following diseases, as you may be able to use Nicorette Supermint, but you will need to consult your doctor first:
- If you have recently suffered a heart attackor stroke.
- If you suffer from chest pain(unstable angina) or rest angina.
- If you suffer from any heart diseasethat affects heart rhythm or frequency (arrhythmia).
- If you have high blood pressurethat is not being controlled with medication.
- If you have heart failureor circulation problems.
- If you have had an allergic reactionwith inflammation of the lips, face, and throat (angioedema) or skin irritation (urticaria). The use of nicotine replacement therapy may sometimes cause this type of reaction.
- If you suffer from severe or moderate liver disease.
- If you suffer from severe kidney disease.
- If you suffer from diabetes.
- If you have hyperthyroidism.
- If you have a pheochromocytoma(adrenal gland tumor).
- If you suffer from duodenal or stomach ulcers.
- If you have esophagitis.
- If you have a history of epilepsy or seizures
Lozenges can pose a choking hazard. Use with caution if you have problems swallowing solids or liquids or if you cough frequently during or after swallowing.
Children and adolescents
Do not administer this medication to children and adolescents.
Using Nicorette Supermint with other medications
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication. This is especially important if you are taking medications that contain:
Theophyllinefor asthma
Tacrinefor Alzheimer's disease
Clozapinefor schizophrenia
Ropinirolefor Parkinson's disease.
Using Nicorette Supermint with food and drinks
Do not eat or drink while using the lozenges. Acidic beverages, such as coffee, juice, or soft drinks, can reduce nicotine absorption. To achieve maximum nicotine absorption, avoid consuming these beverages until 15 minutes before using the lozenge.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medication.
It is very important to quit smoking during pregnancy because it can cause intrauterine growth retardation, premature birth, or stillbirth. It is recommended to quit smoking without using nicotine-containing medications. If you are unable to do so, you may use Nicorette Supermint only after consulting your doctor.
Since nicotine passes into breast milk and can affect the baby, it should only be used during breastfeeding after consulting your doctor. If your doctor has recommended using Nicorette Supermint, the lozenges should be used immediately after breastfeeding the baby and never during the 2 hours prior.
Driving and using machines
Using this medication does not affect your ability to drive or operate machinery.
Nicorette Supermint contains less than 23 mg of sodium (1 mmol) per lozenge, which is essentially "sodium-free".
Nicorette Supermint contains polysorbate, which can cause allergic reactions.
3. How to take Nicorette Supermint
Follow the administration instructions of the medication contained in this leaflet or as indicated by your doctor or pharmacist. If in doubt, ask your doctor or pharmacist.
Minors under 18 years old should not use this medication.
This medication is for oral use. Place the lozenge in your mouth and let it dissolve, releasing nicotine for absorption through the buccal mucosa into the rest of the body.
In all cases, place a lozenge in your mouth and periodically move it from one side of your mouth to the other until it is completely dissolved. This usually takes less than 20 minutes. Do not chew the lozenge or swallow it whole. Do not use more than 15 lozenges per day. If you feel the need to continue taking Nicorette Supermint after 6 months of treatment, you should consult your doctor.
The goal is to completely quit smoking, using Nicorette Supermint lozenges to alleviate the desire to smoke.
Adults over 18 years old
- Start using 8 to 12 lozenges per day. Each time you feel the urge to smoke, use a lozenge, place it in your mouth, and let it dissolve completely.
- Follow this schedule for 6 weeks, then gradually reduce the number of lozenges used each day.
- Once you have reached 1 or 2 lozenges per day, stop using Nicorette Supermint. Once you have stopped, you may feel sudden cravings to smoke. In these cases, you can use a lozenge.
Do not exceed the established dose. Follow the instructions carefully and do not use more than 15 lozenges per day (24 hours).
Do not eat or drink while the lozenge is in your mouth (see section Nicorette Supermint with food and drinks)
In the case of a plastic container:
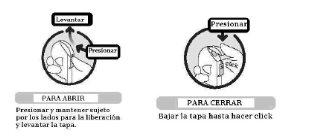
In the case of a cardboard box:

TO OPEN:
- Gently press the tab where it says "press here"
- At the same time, pull the top part of the box upwards with force
TO CLOSE: Press on the top of the box
Consult your doctor if you feel the need to smoke again:
- If you are concerned that you may start smoking again.
- If you have difficulty quitting the lozenges.
If you start smoking again, your doctor may advise you on how to get the best results in future treatments with nicotine replacement therapy (NRT).
If you use more Nicorette Supermint than you should
Nicotine overdose can occur if you smoke while using Nicorette Supermint.
If a child takes Nicorette Supermint, contact your doctor or go to the nearest hospital immediately. Nicotine doses tolerated by adult smokers during treatment can cause poisoning and even deathin small children.
The symptoms of overdose are nausea, vomiting, excessive salivation, stomach pain, diarrhea, sweating, headache, and dizziness, hearing disturbances, and weakness. At high doses, these symptoms can be followed by a drop in blood pressure, weak and irregular pulse, difficulty breathing, extreme fatigue, circulatory collapse, and generalized convulsions.
If you have any other questions about the use of this medication, consult your doctor or pharmacist.
In case of overdose or accidental poisoning, go to a medical center or call the Toxicological Information Service (phone 91 562 04 20), indicating the medication and the amount ingested.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone experiences them.
Effects related to quitting smoking (nicotine withdrawal)
Some of the unwanted effects experienced when quitting smoking may be withdrawal symptoms due to decreased nicotine consumption.
These effects include:
- Irritability, aggression, impatience, or frustration.
- Feeling of anxiety, agitation, or difficulty concentrating.
- Nighttime awakenings or sleep disorders.
- Increased appetite or weight gain.
- Depression.
- Craving to smoke.
- Decreased heart rate.
- Gum bleeding or mouth ulcers.
- Dizziness or mild disorientation.
- Cough, sore throat, nasal congestion, or mucus.
- Constipation.
If you notice any of the following serious side effects, stop using Nicorette Supermint and contact your doctor immediately, as they may be symptoms of a severe allergy (frequency is very rare: may affect up to 1 in 10,000 people):
- Urticaria (skin condition characterized by itching and red spots).
- Swollen face, tongue, or throat.
- Difficulty breathing.
- Difficulty swallowing.
Most of these side effects occur during the initial phases of treatment. During the first few days of treatment, irritation in the mouth and throat may occur; however, in most cases, the patient adapts to continued use.
Other side effects that may occur
Very common: may affect more than 1 in 10 people:
- Cough
- Headache
- Hiccups
- Nausea (feeling of discomfort)
- Irritation of the throat, mouth, and tongue
Common: may affect up to 1 in 10 people:
- Effects at the administration site, such as burning sensation, inflammation of the mouth, or changes in taste perception
- Dry mouth or increased saliva production
- Feeling of indigestion
- Abdominal pain or discomfort
- Vomiting, flatulence, or diarrhea
- Acidity
- Feeling of fatigue
- Feeling of dizziness
- Hypersensitivity (allergy)
- Irritability and feeling of anxiety
Uncommon: may affect up to 1 in 100 people:
- Effects on the nose, such as nasal congestion, sneezing
- Wheezing (bronchospasm), or feeling of needing to breathe more forcefully than normal (dyspnea), throat tightness
- Redness of the skin (flushing), tingling, or increased sweating
- Effects on the mouth, such as tingling, inflammation of the tongue, mouth ulcers, damage to the mouth mucosa, or changes in voice, mouth and throat pain, belching
- Palpitations (unusual sensation of heartbeat), increased heart rate, hypertension.
- Rashes and/or itching (pruritus, urticaria) or redness of the skin
- Depression, feeling of nervousness, abnormal dreams
- Chest discomfort or pain
- Feeling of weakness, feeling of discomfort
Rare: may affect up to 1 in 1,000 people:
- Difficulty swallowing, feeling of numbness in the mouth
- Rapid and erratic heartbeat, which can be treated with appropriate medication
- Hiccups
Frequency not known: the frequency cannot be estimated from the available data:
- Blurred vision, increased tear production (lacrimation)
- Dry throat, stomach discomfort, lip pain
- Redness of the skin.
- Convulsions
Reporting side effects
If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet.
You can also report them directly through the Spanish Medicines Agency's website: www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Nicorette Supermint
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the packaging after EXP. The expiration date is the last day of the month indicated.
For the polypropylene container: Store in the original container to protect from moisture.
In a cardboard box: Store in the original container to protect it from moisture. Use within 3 months of opening the packaging.
Do not reuse the container to store anything else, as the powder from the lozenges may remain in the container and deposit onto the items stored in it.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and unused medications at the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Container Content and Additional Information
Nicorette Supermint Composition
- The active ingredient is Nicotine (in the form of nicotine resin). Each lozenge contains 2 mg of nicotine.
- The other components are: Lozenge core: mannitol (E421), xanthan gum, winterfresh RDE4-149 spray dry flavor (gum arabic (E414), peppermint, menthol, and eucalyptus flavors), anhydrous sodium carbonate (E500)(i), sucralose (E955), potassium acesulfame (E950), magnesium stearate (E470b), Coating: hypromellose (E464), winterfresh RDE4-149 flavor (peppermint, menthol, and eucalyptus flavors), titanium dioxide (E171), sucralose (E955), sepifilm gloss (hypromellose (E464), microcrystalline cellulose (E460), aluminum potassium silicate (E555), titanium dioxide (E171)), potassium acesulfame (E950), polysorbate 80 (E433).
Product Appearance and Container Content
Nicorette Supermint are oval, white or almost white lozenges, printed with an "n" on one side and a "2" on the other side. The size of a lozenge is approximately 14 x 9 x 7 mm.
Container sizes:
Plastic container containing 20 lozenges. Packaged with 1 or 4 containers.
Cardboard container containing 40 lozenges. Packaged with 1 or 2 containers.
Not all container sizes may be marketed
Marketing Authorization Holder and Manufacturer:
Holder
JNTL Consumer Health (Spain), S.L.
C/ Vía de los Poblados 1, Edificio E, planta 3
28033-MADRID
Manufacturer
McNeil AB
Norrbroplatsen 2, SE-251 09
Sweden
Further information about this medicinal product can be obtained from the local representative of the marketing authorization holder:
JNTL Consumer Health (Spain), S.L.
C/ Vía de los Poblados 1, Edificio E, planta 3
28033-MADRID
This medicinal product is authorized in the other Member States under the following names:
Italy: Nicoretteicy
Spain: Nicorette Supermint
Date of the last revision of this leaflet:January 2025
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NICORETTE SUPERMINT 2 mg LOZENGESDosage form: CHEWING GUM, 2 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not requiredDosage form: CHEWING GUM, 4 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not requiredDosage form: CHEWING GUM, 2 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not required
Online doctors for NICORETTE SUPERMINT 2 mg LOZENGES
Discuss questions about NICORETTE SUPERMINT 2 mg LOZENGES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













