
MERIOFERT KIT 900 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar MERIOFERT KIT 900 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Meriofert Kit 900 UIpolvo y disolvente para solución inyectable
Menotropina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- En este prospecto, Meriofert Kit 900 UI de polvo y disolvente para solución inyectable se denomina Meriofert Kit.
Contenido del prospecto
- Qué es Meriofert Kit y para qué se utiliza
- Qué necesita saber antes de empezar a usar Meriofert Kit
- Cómo usar Meriofert Kit
- Posibles efectos adversos
- Conservación de Meriofert Kit
- Contenido del envase e información adicional
1. Qué es Meriofert Kit y para qué se utiliza
- Meriofert Kit se usa para estimular la ovulación en mujeres que no ovulan y que no han
respondido a otro tratamiento (citrato de clomifeno).
- Meriofert Kit se usa para provocar el desarrollo de varios folículos (y, por tanto, de varios óvulos) en mujeres sometidas a un tratamiento de fertilidad.
Meriofert Kit es una gonadotropina menopáusica humana muy purificada, que pertenece a un grupo de medicamentos denominados gonadotropinas.
Cada vial multidosis contiene polvo liofilizado con 900 UI de actividad de hormona foliculoestimulante (FSH) humana y 900 UI de actividad de hormona luteinizante humana (LH).
La gonadotropina menopáusica humana (HMG) es extraída de la orina de mujeres post menopáusicas. Se añade gonadotropina coriónica humana (hCG), extraída de la orina de mujeres embarazadas, para contribuir a la actividad de LH total.
Este medicamento debe usarse bajo la supervisión de un médico.
2. Qué necesita saber antes de empezar a usar Meriofert Kit
Antes de comenzar el tratamiento, se evaluará su fertilidad y la de su pareja.
No use Meriofert Kit
- Si presenta un aumento del tamaño de sus ovarios, o quistes cuya causa no sea un trastorno hormonal (enfermedad ovárica poliquística)
- Si tiene sangrados de origen desconocido
- Si padece cáncer de ovarios, útero o mama
- Si presenta una hinchazón anormal (tumor) de la hipófisis o el hipotálamo (cerebro)
- Si es alérgica a la menotropina o a cualquiera de los demás componentes de este
medicamento (listados en la sección 6).
No debe utilizar este medicamento si sufre menopausia precoz, malformación de los órganos genitales o determinados tumores del útero que impedirían un embarazo normal.
Advertencias y precauciones
Aunque no se han descrito todavía reacciones alérgicas a Meriofert Kit, deberá informar a su médico si padece alguna reacción alérgica a medicamentos similares.
Este tratamiento aumenta el riesgo de padecer una enfermedad denominada síndrome de hiperestimulación ovárica (SHO)(ver Posibles efectos adversos). Si se produce una hiperestimulación ovárica, se suspenderá el tratamiento y deberán evitarse los embarazos. Los primeros signos de hiperestimulación ovárica son dolor en la región inferior del abdomen, náuseas (malestar), vómitos y ganancia de peso. Si se producen dichos síntomas, deberá examinarle un médico lo antes posible. En casos graves, pero raros, pueden agrandarse los ovarios y acumularse líquido en el abdomen o en el pecho.
El medicamento empleado para provocar el desprendimiento definitivo de los óvulos maduros (que contiene hCG) puede aumentar la probabilidad de sufrir un SHO. Por tanto, no es recomendable utilizar hCG en los casos en los que se esté produciendo un SHO y no deberán mantenerse relaciones sexuales durante un mínimo de 4 días, aunque se utilice un método anticonceptivo de barrera.
Cabe destacar que las mujeres con problemas de fertilidad tienen una tasa de abortos superior a la de la población normal.
La frecuencia de embarazos y partos múltiples en las pacientes sometidas a un tratamiento para estimular la ovulación es mayor que en las mujeres que conciben de forma natural. Sin embargo, este riesgo puede reducirse al mínimo si se usa la dosis recomendada.
El riesgo de embarazo extrauterino (embarazo ectópico) es ligeramente mayor en las mujeres con lesiones en las trompas de Falopio.
Los embarazos múltiples y las características de los progenitores sometidos a tratamientos de fertilidad (por ejemplo, la edad de la madre o las características del semen) pueden estar asociados a un aumento del riesgo de defectos de nacimiento.
El tratamiento con Meriofert Kit, al igual que el propio embarazo, puede aumentar la probabilidad de sufrir una trombosis. La trombosis es la formación de un coágulo de sangre en un vaso sanguíneo, la mayoría de las veces en las venas de las piernas o los pulmones.
Comente este hecho con su médico antes de empezar el tratamiento, en especial:
- Si ya sabe que tiene una mayor probabilidad de sufrir una trombosis.
- Si usted o algún familiar cercano ha sufrido alguna vez una trombosis.
- Si padece sobrepeso grave.
Población pediátrica
El medicamento no está indicado para uso pediátrico.
Uso deMeriofert Kit con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Meriofert Kit no se debe utilizar si está embaraza o en período de lactancia.
Conducción y uso de máquinas
La influencia de Meriofert Kit sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
Meriofert Kit contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Meriofert Kit
Dosis y duración del tratamiento
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Mujeres que no ovulan y tienen periodos irregulares o ausencia de menstruación:
Por norma general, la primera inyección de un vial de 75 UI de menotropina se administra durante la primera semana del ciclo tras la menstruación espontánea o inducida.
Posteriormente, se inyecta todos los días la dosis de este medicamento recetada por el médico y se continúa con el tratamiento hasta que se hayan formado uno o más folículos maduros en el ovario. El médico le ajustará la dosis de este medicamento dependiendo de la respuesta ovárica, que se determina por medio de exploraciones clínicas.
En cuanto un folículo alcance la fase de desarrollo necesaria, se interrumpirá el tratamiento con este medicamento y se provocará la ovulación con otra hormona (gonadotropina coriónica, hCG).
La ovulación se produce, por lo general, en un plazo de entre 32 y 48 horas.
En esta fase del tratamiento existe la posibilidad de fertilización. Se le recomendará que mantenga relaciones sexuales todos los días a partir del día anterior a la administración de hCG. Si a pesar de la ovulación no se consigue un embarazo, puede repetirse el tratamiento.
Mujeres sometidas a una estimulación ovárica para el desarrollo folicular múltiple antes de una fertilizaciónin vitrou otras técnicas de reproducción asistida
La finalidad de este método es obtener un desarrollo folicular múltiple simultáneo. El tratamiento comenzará el segundo o tercer día del ciclo con inyecciones de entre 150 y 300 UI de Meriofert Kit. El médico puede optar por administrarle dosis más elevadas si es necesario. La dosis de este medicamento que se inyecta es mayor que la del método empleado para la fertilización natural. El médico se encarga de ajustar la continuación del tratamiento de forma individualizada.
En cuanto se ha desarrollado un número suficiente de folículos, se interrumpe el tratamiento con este medicamento y se provoca la ovulación mediante la inyección de otra hormona (gonadotropina coriónica, hCG).
Cómo administrar Meriofert Kit:
Este medicamento se administra en forma de inyección bajo la piel (por vía subcutánea).
Los viales sólo deben reconstituirse una vez y cada inyección única debe administrarse tan pronto como la dosis necesaria se haya extraído.
Después de aconsejarle e instruirle debidamente, es posible que el médico le pida que se administre la inyección de Meriofert Kit usted misma.
Antes de la primera inyección, su médico debe:
- Permitirle que practique la autoadministración de una inyección subcutánea
- Indicarle los lugares en los que puede administrarse la inyección
- Enseñarle a preparar la solución inyectable
- Explicarle cómo preparar la dosis correcta para la inyección.
Antes de administrarse la inyección de Meriofert Kit, lea detenidamente las siguientes instrucciones.
Dado que este vial contiene medicación para varios días de tratamiento, debe estar seguro que solo extrae la cantidad de medicación que le ha prescrito su médico. Su médico le ha prescrito una dosis de Meriofert Kit en UI (unidades). Para obtener la dosis correcta debe utilizar una de las 12 jeringas de administración graduadas en unidades UI de FSH/LH que se suministran en el envase.
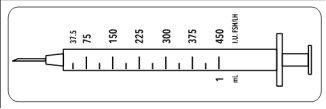
Estas jeringas desechables están destinadas a un solo uso y deben ser desechadas después de la administración de acuerdo con la normativa local en un contenedor adecuado.
Cómo preparar e inyectar 1 vial de Meriofert Kit:
La solución inyección que contiene 900 UI de menotropina debe prepararse justo antes de que esté lista para que se administre la primera dosis. Para ello, añada al vial que contiene el polvo el disolvente para reconstitución de la jeringa precargada (incluida en el envase).
Prepare una superficie limpia y lávese las manos con jabón y agua tibia. Es importante que sus manos y los artículos que utilice estén lo más limpios posible.
Coloque sobre la superficie los artículos siguientes:
- el vial de polvo de Meriofert Kit
- la jeringa precargada con disolvente para la reconstitución
- la aguja para preparar la reconstitución
- una jeringa desechable de administración subcutánea con aguja prefijada graduada en unidades FSH/LH
- un bastoncillo de algodón con alcohol
- algodón y solución desinfectante (no incluidos en el envase)
RECUERDE:
- Desinfectar el tapón de goma del vial que contiene la solución reconstituida con algodón y desinfectante (esto es, solución alcohólica) y dejar secar antes de la reconstitución y de cada administración.
- No retire la lengüeta de apoyo (pieza blanca) de la jeringa precargada, ya que evita la extracción involuntaria del pistón y mejora el manejo de la jeringa durante la inyección.
Reconstitución de la solución inyectable
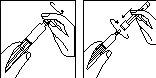
Preparación de la jeringa precargada:
 1.
1.
- Retire el capuchón de la jeringa precargada con disolvente; acople la aguja de reconstitución con el tapón protector todavía en la jeringa.
- Coloque con cuidado la jeringa sobre una superficie limpia.
Preparación del vial:
 2.
2.
- Retire el tapón abatible de plástico coloreado del vial empujándolo suavemente con el pulgar hacia arriba.
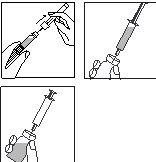 Limpie la zona del tapón de goma con un algodón y una solución desinfectante y déjelo secar.
Limpie la zona del tapón de goma con un algodón y una solución desinfectante y déjelo secar.
3.
- Coja la jeringa, retire el capuchón protector de la aguja y presione la aguja a través del centro del tapón de goma del vial.
- Empuje con firmeza el émbolo para vaciar toda la solución sobre el polvo.
- Al añadir el disolvente, se crea una ligera sobrepresión en el vial. Por lo tanto, suelte el émbolo de la jeringa para que suba por sí mismo durante unos 10 segundos. Esto eliminará la sobrepresión en el vial.
NO AGITE la solución reconstituida, hágala girar suavemente hasta obtener una solución transparente. Por lo general, el medicamento se disuelve inmediatamente.
Compruebe que la solución reconstituida es transparente.
Antes de la inyección:
- Compruebe que la solución reconstituida sea trasparente, incolora y sin partículas. NO USAR si la solución contiene partículas, está turbia o no es incolora.
- Limpie la zona del tapón de goma con un algodón y una solución desinfectante.
Preparación de la inyección:
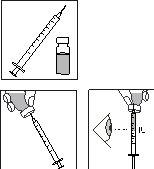 4.
4.
- Coja una de las jeringas desechables con aguja prefijada, retire el capuchón protector de la aguja e inserte la aguja verticalmente en el centro de la parte superior del vial.
- Empuje el émbolo hasta que esté completamente presionado.
- Invierta el vial. Asegúrese de que la aguja está debajo de la superficie del medicamento y extraiga la dosis prescrita de medicamento en la jeringa de administración.
- Retire la aguja del vial. Sostenga la jeringa con la aguja apuntando hacia arriba y golpee suavemente el lado de la jeringa para forzar cualquier burbuja de aire hacia arriba.
- Empuje el émbolo lentamente hasta que aparezca una gota de líquido en la punta de la aguja.
RECUERDE: debido a que el vial contiene medicación para varios días de tratamiento, debe asegurarse que sólo retira la cantidad de medicación que le ha prescrito el médico.
Administración de la inyección
Zona de inyección:
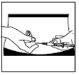 Su médico o enfermero ya le habrá explicado en qué parte del cuerpo debe inyectarse el medicamento. Los lugares habituales son el muslo o la pared inferior del abdomen bajo el ombligo.
Su médico o enfermero ya le habrá explicado en qué parte del cuerpo debe inyectarse el medicamento. Los lugares habituales son el muslo o la pared inferior del abdomen bajo el ombligo.
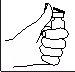
- Limpie la zona de inyección con un bastoncillo de algodón con alcohol.
- Pellizque y apriete con firmeza la piel. Con la otra mano, introduzca la aguja con un movimiento seco y rápido, formando un ángulo de 45° o 90°.
Inyección de la solución:
- Inyecte la jeringa bajo la piel tal como le indicaron. No la inyecte directamente en una vena. Empuje el émbolo lentamente y sin interrupciones, para que la solución se inyecte correctamente y los tejidos cutáneos no sufran daños.
Tómese el tiempo que necesite para inyectar el volumen de solución que le han recetado.
Extraiga rápidamente la aguja y presione la zona de inyección con un algodón con desinfectante. Masajee suavemente la zona (mientras mantiene la presión), esto ayuda a dispersar el medicamento y alivia las posibles molestias.
Siguientes inyecciones:
Repetir desde el paso 4 en adelante para las siguientes inyecciones con la solución reconstituida de Meriofert Kit.
Si usa más MeriofertKitdel que debe
Se desconocen los efectos de la sobredosis de este medicamento, no obstante, cabría esperar que se produjera un síndrome de hiperestimulación ovárica (ver Posibles efectos adversos). Si usa más medicamento del que debe, consulte a su médico o enfermero.
Si olvidó usar Meriofert Kit
Úselo en el plazo en que le tocaría normalmente la siguiente inyección. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Meriofert Kit
No lo interrumpa por iniciativa propia. Consulte siempre a su médico antes de dejar de tomar este medicamento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
El siguiente efecto adverso es importante y exigirá una intervención inmediata si lo padece. Deberá dejar de tomar este medicamento y acudir al médico de inmediato si se produce lo siguiente:
Frecuentes (puede afectar hasta 1 de cada 10 personas):
- Síndrome de hiperestimulación ovárica (los síntomas comprenden la formación de quistes ováricos o el aumento del tamaño de quistes existentes, dolor en la parte inferior del estómago, sensación de sed y náuseas con vómitos ocasionales, evacuación de pequeñas cantidades de orina concentrada y ganancia de peso) (ver sección 2 para obtener información adicional).
También se han comunicado los siguientes efectos adversos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Cefalea
- Hinchazón del estómago o meteorismo
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Dolor o molestia abdominal
- Dolor pélvico
- Dolor de espalda
- Sensación de pesadez
- Molestia en mama
- Mareo
- Sofocos
- Sed
- Sensación de enfermedad
- Cansancio
- Malestar general
- Reacciones en la zona de inyección, tales como dolor e inflamación
Raras (pueden afectar hasta 1 de cada 1.000 personas):
- Torsión ovárica (rotación del ovario que causa un dolor de gran intensidad en la parte inferior del abdomen)
- Tromboembolia (formación de un coágulo en un vaso sanguíneo que se desprende y es transportado por el torrente sanguíneo para bloquear otro vaso).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Meriofert Kit
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el embalaje exterior, el vial y la jeringa precargada de disolvente después de CAD. Si la fecha de caducidad se indica como mes/año, la fecha de caducidad es el último día del mes que se indica.
Antes de la reconstitución: Conservar en nevera (entre 2ºC y 8ºC).
Tras la reconstitución, la solución puede conservarse durante un máximo de 28 días a no más de 25º C.
No congelar antes o después de la reconstitución.
No utilice este medicamento si observa que la solución no es transparente. Tras la reconstitución, la solución debe ser transparente e incolora.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE  de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Meriofert Kit
El principio activo es menotropina.
Cada vial multidosis contiene polvo liofilizado con 900 UI de actividad de hormona foliculoestimulante (FSH) humana y 900 UI de actividad de hormona luteinizante (LH) humana.
La gonadotropina menopáusica humana (HMG) es extraída de la orina de mujeres post menopáusicas. Se añade gonadotropina coriónica humana (hCG), extraída de la orina de mujeres embarazadas, para contribuir a la actividad de LH total.
Los demás componentes son
Polvo: lactosa monohidrato, polisorbato 20, dihidrogenofosfato de sodio dihidrato, ácido fosfórico e hidróxido de sodio.
Disolvente: metacresol y agua para preparaciones inyectables.
Aspecto de Meriofert Kit y contenido del envase
Polvo: torta o polvo blanco liofilizado.
Disolvente: solución transparente e incolora.
Meriofert Kit se presenta como polvo y disolvente para solución inyectable.
Un estuche contiene:
- 1 vial de polvo de Meriofert Kit
- 1 jeringa precargada con disolvente para la reconstitución
- 1 aguja para la reconstitución
- 12 bastoncillos de algodón con alcohol para las inyecciones múltiples
- 12 jeringas desechables con agujas prefijadas para las inyecciones múltiples
Titular de la autorización de comercialización y responsable de fabricación
Titular de la autorización de comercialización
IBSA FARMACEUTICI ITALIA SRL
Via Martiri di Cefalonia 2
26900 Lodi, Italia
Responsable de la fabricación
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Italia
o (para Reino Unido/Irlanda del Norte)
IBSA Pharma Limited
Units 4-6
Colonial Business Park
Colonial Way
Watford D24 4PR
Reino Unido
Puede solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (España)
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo y en el Reino Unido (Irlanda del Norte) con los siguientes nombres (las concentraciones y formas farmacéuticas son idénticas en todos los países, sólo cambian los nombres comerciales):
Austria: Meriofert PFS
Bélgica: Fertinorm Kit
Bulgaria: Meriofert PFS
Chipre: Meriofert PFS
República Checa: Meriofert Set
Dinamarca: Meriofert Set
Estonia: Meriofert Set
Finlandia: Meriofert Set
Francia: Fertistartkit
Grecia: Meriofert
Hungria: Meriofert Kit
Italia: Meriofert
Letonia: Meriofert Set
Lituania: Meriotert Set
Luxemburgo: Fertinorm Kit
Noruega: Meriofert Set
Polonia: Mensinorm Set
Rumania: Meriofert PFS
Eslovaquia: Meriofert Kit
España: Meriofert Kit
Suecia: Meriofert Set
Países Bajos: Meriofert spuit
Reino Unido: Meriofert PFS
Fecha de la última revisión de este prospecto: Mayo 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MERIOFERT KIT 900 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, -Principio activo: human menopausal gonadotrophinFabricante: Angelini Pharma Espana S.L.Requiere recetaForma farmacéutica: INYECTABLE, 1200 UIPrincipio activo: human menopausal gonadotrophinFabricante: Ferring S.A.Requiere recetaForma farmacéutica: INYECTABLE, 1.200 UIPrincipio activo: human menopausal gonadotrophinFabricante: Ferring S.A.U.Requiere receta
Médicos online para MERIOFERT KIT 900 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MERIOFERT KIT 900 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






