
LENZETTO 1,53 MG/DOSIS SOLUCION PARA PULVERIZACION TRANSDERMICA


Cómo usar LENZETTO 1,53 MG/DOSIS SOLUCION PARA PULVERIZACION TRANSDERMICA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para la paciente
Lenzetto 1,53 mg/dosis, solución para pulverización transdérmica
estradiol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Lenzetto y para qué se utiliza
- Qué necesita saber antes de empezar a usar Lenzetto
- Cómo usar Lenzetto
- Posibles efectos adversos
- Conservación de Lenzetto
- Contenido del envase e información adicional
1. Qué es Lenzetto y para qué se utiliza
Lenzetto es una Terapia Hormonal Sustitutiva (THS). Contiene la hormona femenina estrógeno. Lenzetto se utiliza en mujeres postmenopáusicas cuando han transcurrido al menos 6 meses desde su último periodo menstrual natural.
Lenzetto también puede utilizarse en mujeres que han tenido una cirugía para extirpar sus ovarios ya que esto provoca una menopausia instantánea.
Lenzetto es una solución para pulverización que contiene pequeñas cantidades de un medicamento llamado estradiol. Cuando se pulveriza sobre la piel como se indica, pasa a través de la piel al torrente sanguíneo.
Lenzetto se utiliza para:
Alivio de los síntomas que ocurren tras la menopausia
Durante la menopausia, la cantidad de estrógeno producido por el cuerpo de la mujer disminuye. Esto puede causar síntomas como calor en la cara, cuello y pecho ("sofocos"). Lenzetto alivia estos síntomas tras la menopausia. Sólo se le recetará Lenzetto si sus síntomas dificultan seriamente su vida cotidiana.
Lenzetto está indicado para tratar los síntomas de la deficiencia de estrógeno tras la menopausia, cuando la menstruación ya no se produce tras la menopausia. Los síntomas de la deficiencia de estrógenos incluyen sofocos (olas repentinas de calor y sudoración en todo el cuerpo), problemas para dormir, irritabilidad y sequedad de la vagina.
La experiencia en el tratamiento de mujeres mayores de 65 años es limitada.
Lenzetto no es un anticonceptivo.
2. Qué necesita saber antes de empezar a usar Lenzetto
Historia médica y revisiones regulares:
El uso de THS conlleva riesgos que necesitan ser considerados al decidir si se empieza a utilizarla, o si se continúa utilizando.
La experiencia en el tratamiento de mujeres con menopausia prematura (debido a fallo ovárico o cirugía) es limitada. Si usted tiene una menopausia prematura, los riesgos de usar THS pueden ser diferentes. Por favor, consulte a su médico.
Antes de empezar (o retomar) la THS, su médico le preguntará por su historia clínica personal y familiar. Su médico podrá decidir llevar a cabo una exploración física. Ésta puede incluir un examen de sus mamas y/o un examen interno, si fuera necesario.
Una vez empezado el tratamiento con Lenzetto, debe visitar a su médico para realizar revisiones periodicas (al menos una vez al año). En estas revisiones, comente con su médico los beneficios y riesgos de continuar con Lenzetto.
Sométase periodicamente a las revisiones de mama que le recomiende su médico.
No use Lenzetto
Si cualquiera de las siguientes situaciones le aplica a usted. Si no está segura acerca de alguno de los puntos a continuación, consulte a su médicoantes de usar Lenzetto.
No use Lenzetto
- si tiene o ha tenido cáncer de mama, o si sospecha que lo tiene;
- si tiene cáncer sensible a los estrógenos, tales como cáncer de la capa interna del útero (endometrio), o si sospecha que lo tiene;
- si tiene cualquier sangrado vaginal inexplicable;
- si tiene un engrosamiento excesivo de la capa interna del útero(hiperplasia endometrial) que no está siendo tratado;
- si tiene o ha tenido un coagulo sanguíneo en una vena(trombosis), como en las piernas (trombosis venosa profunda) o en los pulmones (embolismo pulmonar);
- si tiene un trastorno de la coagulación sanguínea (tales como deficiencia de proteína C, proteína S o de antitrombina);
- si tiene o ha tenido recientemente una enfermedad causada por coágulos sanguíneos en las arterias, tales como un ataque al corazón, ictuso angina;
- si tiene, o ha tenido alguna vez, una enfermedad hepáticay sus tests de función hepática no se han normalizado;
- si tiene una enfermedad rara de la sangre denominada "porfiria" que se transmite en familias (hereditaria);
- si es alérgicoal estradiol o a alguno de los demás componentes de Lenzetto (incluidos en la sección 6).
Si cualquiera de las anteriores condiciones aparece por primera vez durante el uso de este medicamento, interrumpa el tratamiento enseguida y consulte con su médico inmediatamente.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Lenzetto.
Informe a su médico si ha tenido alguna vez alguno de los siguientes problemas, antes de comenzar el tratamiento, ya que pueden aparecer de nuevo o empeorar durante el tratamiento con Lenzetto. En ese caso, debería acudir a su médico con mayor frecuencia para someterse a revisiones:
- fibromas en su útero;
- crecimiento de la capa interna del útero fuera del útero (endometriosis) o antecedentes de crecimiento excesivo de la capa interna del útero (hiperplasia endometrial);
- aumento del riesgo de desarrollar coágulos sanguíneos (ver “Coagulo sanguíneo en una vena (trombosis)”);
- aumento del riesgo de contraer un cáncer dependiente de estrógeno (como tener una madre, hermana o abuela que ha tenido cáncer de mama);
- presión sanguínea alta;
- un trastorno hepático, tales como tumor benigno en el hígado;
- diabetes;
- piedras en la vesícula biliar;
- migraña o dolor de cabeza intenso;
- una enfermedad del sistema inmunitario que afecta a varios órganos del cuerpo (Lupus eritematoso sistémico, LES);
- epilepsia;
- asma;
- una enfermedad que afecta al tímpano y al oído (otosclerosis);
- un nivel muy elevado de grasa en su sangre (triglicéridos);
- retención de líquidos debida a problemas cardiacos o renales;
- angioedema hereditario y adquirido.
Interrumpa el tratamiento conLenzettoy acuda a un médico inmediatamente
Si observa cualquiera de los siguientes síntomas mientras usa THS:
- cualquiera de las situaciones mencionadas en la sección “NO use Lenzetto”;
- coloración amarillenta de su piel o del blanco de sus ojos (ictericia). Estos pueden ser signos de enfermedad hepática;
- hinchazón de la cara, lengua o garganta y dificultad para tragar o urticaria acompañados de dificultad para respirar, que sugieren un angioedema;
- aumento importante de su presión arterial (los síntomas pueden ser dolor de cabeza, cansancio, mareos);
- dolores de cabeza de tipo migrañoso que ocurren por primera vez;
- si se queda embarazada;
- si aprecia signos de un coágulo sanguíneo, como:
- hinchazón dolorosa y enrojecimiento de las piernas
- dolor de pecho repentino
- dificultad para respirar.
Para más información, ver ‘Coágulo sanguíneo en una vena (trombosis)’.
Nota: Lenzetto no es un anticonceptivo. En el caso de que hayan transcurrido menos de 12 meses desde su última menstruación o tenga menos de 50 años, aún puede necesitar medidas anticonceptivas adicionales para prevenir un embarazo. Pida consejo a su médico.
THS y cáncer
Engrosamiento excesivo de la capa interna del útero (hiperplasia endometrial) y cáncer de la capa interna del útero (cáncer endometrial).
La THS con solo estrógeno aumentará el riesgo de engrosamiento excesivo de la capa interna del útero (hiperplasia endometrial) y de cáncer de la capa interna del útero (cáncer endometrial).
Tomar un progestágeno además del estrógeno durante al menos 12 días de cada ciclo de 28 días le protege de este riesgo adicional. Por lo que su médico le prescribirá por separado un progestágeno si todavía tiene útero. Si le han extirpado el útero (histerectomía), consulte con su médico si puede usar este medicamento sin progestágeno de manera segura.
En mujeres con útero y no tratadas con THS, de media, 5 de cada 1.000 serán diagnosticadas de cáncer endometrial entre los 50 y 65 años.
En mujeres de edades comprendidas entre los 50 y 65 años, con útero y en tratamiento con THS de solo estrógenos, entre 10 y 60 mujeres de cada 1.000 serán diagnosticadas de cáncer endometrial (esto es, entre 5 y 55 casos adicionales), dependiendo de la dosis y del tiempo de tratamiento.
Lenzetto contiene una dosis más alta de estrógeno que otros productos de THS con solo estrógeno. Se desconoce el riesgo de cáncer endometrial cuando se utiliza Lenzetto junto con un progestágeno.
Sangrado inesperado
Tendrá un sangrado una vez al mes (llamado sangrado por deprivación) mientras utiliza Lenzetto si se combina con un progestágeno de dosis secuencial. Pero, si tiene sangrado inesperado o pequeñas pérdidas (manchado) además de su sangrado mensual, que:
- continúa pasados los 6 primeros meses;
- comienzan después de haber utilizado Lenzetto durante más de 6 meses;
- continúa después de haber dejado de usar Lenzetto;
acuda a su médico lo antes posible.
Cáncer de mama
Está demostrado que la terapia hormonal sustitutiva (THS) combinada estrógeno-progestágeno o la THS con solo estrógeno aumenta el riesgo de cáncer de mama. El riesgo adicional depende del tiempo durante el cual se use la THS. El riesgo adicional se hace evidente dentro de los 3 años de uso. Después de suspender la THS, el riesgo adicional disminuirá con el tiempo, pudiendo persistir durante 10 años o más si ha utilizado la THS durante más de 5 años.
Comparación
En mujeres de 50 a 54 años de edad que no usan THS, como media, de 13 a 17 de cada 1.000 serán diagnosticadas de cáncer de mama en un periodo de 5 años.
En mujeres de 50 años que comienzan a usar THS con solo estrógenos durante 5 años, se producirán 16 – 17 casos por cada 1.000 usuarias (es decir, de 0 a 3 casos más).
En mujeres de 50 años que comienzan a usar THS con estrógeno-progestágeno durante 5 años, se producirán 21 casos por cada 1.000 usuarias (es decir, de 4 a 8 casos más).
En mujeres de 50 a 59 años que no usan THS se diagnosticará cáncer de mama en una media de 27 de cada 1.000 en un periodo de 10 años.
En las mujeres de 50 años que empiecen a usar THS con solo estrógeno durante 10 años, se producirán 34 casos de cada 1.000 usuarias (es decir, 7 casos más).
En mujeres de 50 años que comienzan a usar THS con estrógeno-progestágeno durante 10 años, se producirán 48 casos por cada 1.000 usuarias (es decir, 21 casos más).
Examine sus mamas regularmente. Acuda a su médico si detecta cualquier cambio, como:
- formación de hoyuelos en la piel
- cambios en el pezón
- cualquier bulto que pueda ver o notar.
Además, se le aconseja unirse a los programas de detección mamográfica cuando se le ofrezcan. Para la mamografía, es importante que informe al enfermero/profesional sanitario que vaya a realizar la radiografía que utiliza THS, ya que este medicamento puede aumentar la densidad de sus mamas, lo que puede afectar el resultado de la mamografía. Cuando aumenta la densidad de la mama, es posible que la mamografía no detecte todos los bultos.
Cáncer de ovario
El cáncer de ovario es poco frecuente, mucho menos frecuente que el cáncer de mama. El uso de THS con estrógenos solos o con combinación de estrógenos-progestágenos se ha asociado con un riesgo ligeramente mayor de cáncer de ovario.
El riesgo de cáncer de ovario varía con la edad. Por ejemplo, en mujeres de entre 50 y 54 años de edad que no usan THS, se han observado alrededor de 2 casos de cáncer de ovario por cada 2.000 mujeres en un período de 5 años. En mujeres en tratamiento con THS durante 5 años, se han observado alrededor de 3 casos por cada 2.000 pacientes (es decir, alrededor de 1 caso adicional).
Efecto de la THS sobre el corazón y la circulación
Coágulos sanguíneos en una vena (trombosis)
El riesgo de coágulos sanguíneos en las venases aproximadamente de 1,3 a 3 veces mayor en las usuarias de THS que en las no usuarias, especialmente durante el primer año de tratamiento.
Los coágulos sanguíneos pueden ser graves, y si uno se desplaza a los pulmones, puede causar dolor en el pecho, dificultad para respirar, desmayos o incluso la muerte.
Tiene más probabilidades de desarrollar un coágulo sanguíneo en sus venas a medida que envejece y si padece alguna de las siguientes situaciones. Informe a su médico si padece alguna de las situaciones siguientes:
- no puede caminar durante mucho tiempo debido a cirugía mayor, lesión o enfermedad (ver también la sección 3, “Si necesita cirugía”).
- tiene sobrepeso grave (IMC>30 kg/m2).
- tiene algún problema de coagulación sanguínea que necesite tratamiento a largo plazo con un medicamento utilizado para prevenir coágulos sanguíneos.
- si alguno de sus familiares cercanos ha tenido alguna vez un coágulo sanguíneo en una pierna, pulmón u otro órgano.
- padece lupus eritematoso sistémico (LES).
- tiene cáncer.
Para conocer los signos de coágulo sanguíneo, vea “Interrumpa el tratamiento conLenzettoy acuda a un médico inmediatamente”.
Considerando a las mujeres de 50 años que no utilizanTHS, de media, en un periodo de 5 años, se espera que de 4 a 7 de cada 1.000 tengan un coágulo sanguíneo en una vena.
En mujeres de 50 años que han estado utilizando THS con estrógeno-progestágeno combinado durante 5 años, habrá de 9 a 12 casos por cada 1.000 usuarias (esto es, 5 casos adicionales).
En mujeres de 50 años a las que se ha extirpado el útero y han estado utilizando THS con solo estrógeno durante 5 años, habrá de 5 a 8 casos por cada 1.000 usuarias (esto es, 1 caso adicional).
Enfermedad cardiaca (infarto)
No hay evidencia de que la THS prevenga un infarto.
Mujeres mayores de 60 años que utilizan THS con estrógeno-progestágeno combinado son ligeramente más propensas a desarrollar una enfermedad cardiaca que aquellas que no utilizan THS.
En el caso de mujeres a las que se les ha extirpado el útero y que solo utilizan terapia con estrógeno, no existe un mayor riesgo de desarrollar una enfermedad cardiaca.
Ictus
El riesgo de sufrir un ictus es alrededor de 1,5 veces mayor en mujeres tratadas con THS que en las no tratadas. El número de casos adicionales de ictus debidos al uso de THS aumentará con la edad.
Comparación: En el caso de mujeres de 50 años que no utilizan THS, de media, se espera que 8 de cada 1.000 sufran un ictus durante un periodo de 5 años. En mujeres de 50 años que utilizan THS, se producirán 11 casos de cada 1.000 usuarias en 5 años (esto es, 3 casos adicionales).
Otras condiciones
La THS no previene la pérdida de memoria. Existe cierta evidencia de un mayor riesgo de pérdida de memoria en mujeres que empiezan a utilizar la THS después de los 65 años. Consulte a su médico.
Niños
El aerosol de estradiol puede transferirse accidentalmente de la piel donde se ha pulverizado a otras personas. No permita que otras personas, especialmente niños, entren en contacto con el área expuesta de su piel y cubra la zona, si es necesario, después de que el spray se haya secado. Si un niño entra en contacto con la zona de la piel sobre la que se pulverizó estradiolLenzetto, lave la piel del niño con agua y jabón lo antes posible. Debido a la transferencia de estradiol, los niños pequeños pueden mostrar signos de pubertad no esperados (por ejemplo, desarrollo de las mamas). En la mayoría de los casos, los síntomas desaparecerán cuando los niños dejen de estar expuestos al aerosol de estradiol.
Póngase en contacto con a su médico si observa cualquier signo y síntoma (desarrollo de los senos u otros cambios sexuales) en un niño que pueda haber estado expuesto accidentalmente al spray de estradiol.
Otros medicamentos y Lenzetto
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Algunos medicamentos pueden interferir con el efecto de Lenzetto. Esto puede provocar sangrados irregulares. Esto ocurre con los siguientes medicamentos:
- Medicamentos para la epilepsia(como fenobarbital, fenitoína y carbamazepina)
- Medicamentos para la tuberculosis(como rifampicina, rifabutina)
- Medicamentos para la infección por VIH(como nevirapina, efavirenz, ritonavir y nelfinavir)
- Medicamentos (tradicionales) a base de plantas que contengan hierba de San Juan(Hypericum perforatum)
La THS puede afectar la forma en que actúan otros medicamentos:
- Un medicamento para la epilepsia (lamotrigina), ya que podría aumentar la frecuencia de las convulsiones;
- Los medicamentos para el virus de la hepatitis C (VHC) (p. ej., pauta combinada para el VHC ombitasvir/paritaprevir/ritonavir con y sin ºdasabuvir o glecaprevir/pibrentasvir) pueden provocar elevaciones en los resultados sanguíneos de la función hepática (aumento de la enzima hepática ALT) en mujeres que utilizan AHC con etinilestradiol. Lenzetto contiene estradiol en lugar de etinilestradiol. Se desconoce si se puede producir un aumento de la enzima hepática ALT cuando se utiliza Lenzetto con esta pauta combinada para el VHC.
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluidos medicamentos sin receta, medicamentos a base de hierbas u otros productos naturales.
Pruebas de laboratorio
Si necesita un análisis de sangre, comente a su médico o al personal de laboratorio que está utilizando Lenzetto, porque este medicamento puede afectar a los resultados de algunos análisis.
Embarazo y lactancia
Lenzetto es para uso únicamente en mujeres postmenopáusicas. Si se queda embarazada, interrumpa el tratamiento con Lenzetto y contacte con su médico.
No utilice Lenzetto mientras esté en periodo de lactancia.
Conducción y uso de máquinas
Lenzetto no tiene efectos conocidos sobre la capacidad para conducir o utilizar máquinas.
Lenzetto contiene alcohol
Este medicamento contiene 65,47 mg de alcohol (etanol) en cada dosis que equivale a 72,74% p/v. Puede causar sensación de ardor en piel lesionada.
Los productos a base de alcohol son inflamables. Mantener alejado del fuego. Evite llamas abiertas, cigarrillos encendidos o el uso de aparatos que son fuente de calor (p. ej., secadores de pelo) mientras aplica el aerosol sobre su piel, hasta que la pulverización del medicamento se haya secado.
3. Cómo usar Lenzetto
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico le recetará la dosis más baja para tratar sus síntomas durante el tiempo necesario.
Durante el tratamiento, su médico puede ajustar la dosis de acuerdo a sus necesidades individuales. Hable con su médico si considera que la dosis es demasiado fuerte o insuficiente.
Si no se ha sometido a una histerectomía (intervención quirúrgica para extipar el útero), su médico le dará unos comprimidos que contienen otra hormona llamada progestágeno para contrarrestar los efectos del estrógeno en el revestimiento del útero. Su médico le explicará cómo tomar estos comprimidos. Al final del periodo de tratamiento con progestágenos puede producirse un sangrado por deprivación (ver sección "sangrado inesperado").
Si necesita una intervención quirúrgica
Si va a someterse a una intervención quirúrgica, informe al cirujano de que está utilizando Lenzetto. Puede que necesite interrumpir el uso de Lenzetto alrededor de 4 a 6 semanas antes de la operación para reducir el riesgo de un coágulo sanguíneo (ver la sección 2, Coágulos sanguíneos en una vena). Pregúnte a su médico cuándo puede empezar a utilizar Lenzetto de nuevo.
Donde aplicar Lenzetto
El aerosol se debe aplicar sobre la piel seca y sana de la parte interna del antebrazo. Si no es posible, debe aplicarse en la parte interna del muslo.
No aplique Lenzetto en las mamas ni en ninguna zona cercana a los senos.
Cómo aplicar Lenzetto
Antes de usar un nuevo aplicador por primera vez, se debe cebar la bomba pulverizando tres veces con la tapa puesta:El envase se debe mantener en posición vertical como se muestra en la Figura 1. Con la tapa puesta, presione el botón hacia abajo tres veces con su dedo pulgar o índice.
El medicamento ya está listo para su uso.
NO prepare el aplicador antes de cada dosis; prepárelo solo una vez antes de empezar a utilizar un nuevo envase. Si olvidó una o más dosis, prepare el aplicador según las instrucciones de la sección “Si olvidó usar Lenzetto”.
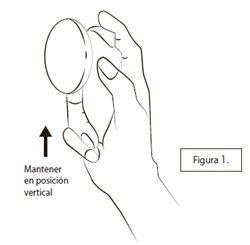
Asegúrese de que la piel donde quiere pulverizar el medicamento está sana, limpia y seca.
Cómo aplicar su dosis diaria.
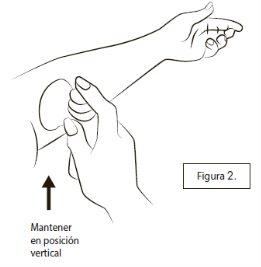
Para aplicar su dosis diaria, quite la tapa de plástico, sostenga el envase en posición vertical y apoye el cono de plástico plano contra la piel (Figura 2).
Puede que tenga que mover su brazo o mover el cono sobre el brazo de manera que el cono quede plano sobre la piel y no haya huecos entre el cono y la piel.
Presione el botón hacia abajo una vez. Se debe presionar a fondo y mantener presionado antes de soltar.
Si necesita otra pulverización, mueva el cono a lo largo de su brazo de manera que esté al lado del área que ya ha pulverizado. Presione el botón hacia abajo una vez.
Si necesita una tercera pulverización, mueva de nuevo el cono a lo largo de su brazo y presione el botón hacia abajo una vez.
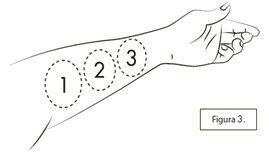
Si la segunda o tercera pulverización no cabe en la misma parte interna del antebrazo, puede pulverizar también en la parte interna de su otro antebrazo. Si tiene problemas para poner el cono en la parte interna del antebrazo como se muestra en la Figura 3, o si le resulta difícil usarlo en los antebrazos, también puede pulverizar sobre la superficie interna del muslo.
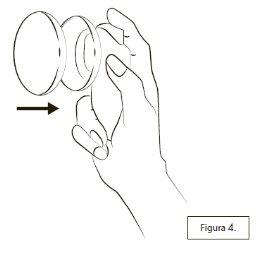
Cuando haya terminado de utilizar Lenzetto, ponga siempre la tapa en el envase (Figura 4).
Si el medicamento se utiliza según las instrucciones, independientemente de las distintas formas o patrón de administración sobre la piel, cada pulverización liberará la misma cantidad de principio activo.
Deje secar la pulverización durante al menos 2minutos antes de vestirse y al menos 60minutos antes de bañarse o lavarse. Si el spray de Lenzetto entra en contacto con otra zona de la piel como las manos, lávese esa zona de la piel con agua y jabón de inmediato.
Lenzetto no se puede utilizar sobre piel lesionada o dañada.
No masajee ni frote Lenzetto sobre la piel.
No permita que otras personas toquen la zona de la piel en la que se ha aplicado el spray hasta que éste se haya secado y cúbralo con ropa 2 minutos después de la aplicación, si es necesario.Si otra persona (especialmente un niño) toca accidentalmente el área de su piel donde ha pulverizado Lenzetto, dígale a esa persona que se lave el área de su piel con agua y jabón de inmediato.
Cuanto Lenzetto debe usar
Su médico probablemente le recomendará inicialmente la dosis más baja (una pulverización al día) y usted deberá hablar con su médico sobre cómo le está funcionando el medicamento. Si es necesario, su médico puede aumentar la dosis a dos pulverizaciones al día. La dosis máxima diaria es de 3 pulverizaciones.
Con qué frecuencia debe usar Lenzetto
Debe aplicar el número total de pulverizaciones (dosis) que su médico le ha prescrito a la misma hora todos los días.
El periodo de tiempo que debe estar usando Lenzetto
Hable con su médico cada 3-6 meses sobre durante cuánto tiempo debe utilizar Lenzetto. Solo debe usar Lenzetto durante el tiempo que lo necesite para aliviar los sofocos asociados con la menopausia.
Otra información útil
Los protectores solares pueden alterar la absorción del estrógeno de Lenzetto.
Evite utilizar protector solar en la parte de la piel donde pretenda pulverizar. No obstante, si necesita usar protector solar, deberá aplicarlo al menos una hora antes del uso de Lenzetto.
Lenzetto debe utilizarse con precaución en condiciones de temperatura extrema, como en la sauna o al tomar el sol.
Hay datos limitados que sugieren que la velocidad y grado de absorción de Lenzetto se pueden reducir en mujeres con sobrepeso y obesidad. Consulte con su médico. Durante el tratamiento su médico puede ajustar la dosis según sus necesidades individuales.
Si usa más Lenzetto del que debe
Si usa más Lenzetto del que debe, o si los niños han estado usando el medicamento por accidente, póngase en contacto con su médico o con el hospital para que le den una opinión sobre el riesgo y consejo sobre las medidas que deban tomarse.
Si usa más Lenzetto del que debe, puede sentirse mal, vomitar y tener un sangrado por deprivación (sangrado vaginal inusual).
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad utilizada.
Si olvidó usar Lenzetto
Si olvidó usar Lenzetto a su hora habitual, aplíquese el medicamento en cuanto se acuerde y, al día siguiente, utilícelo como lo haría normalmente. Si es casi la hora de su próxima dosis, espere y aplique la siguiente dosis como lo haría normalmente. Si olvida una o más dosis, será necesario una primera pulverización con la tapa protectora puesta. No use una dosis doble para compensar las dosis olvidadas.
Olvidar una dosis puede aumentar la probabilidad de sangrado intermensual y manchado.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
Si interrumpe el tratamiento con Lenzetto
Su médico le explicará cómo debe interrumpir el tratamiento con este medicamento cuando su tratamiento haya terminado.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Las siguientes enfermedades se han notificado con más frecuencia en mujeres que usan THS en comparación con las mujeres que no usan THS:
- cáncer de mama;
- crecimiento anormal o cáncer de la capa interna del útero (hiperplasia o cáncer endometrial);
- cáncer de ovario;
- coágulos sanguíneos en las venas de las piernas o pulmones (tromboembolismo venoso);
- enfermedad cardíaca:
- ictus;
- enfermedad de la vesícula biliar;
- presión sanguínea alta;
- problemas en el hígado;
- niveles altos de azúcar en sangre;
- probable pérdida de memoria si la THS se inicia después de los 65 años.
Para más información sobre estos efectos adversos, ver la sección 2.
Algunos efectos adversos pueden ser graves
Los siguientes síntomas necesitan atención médica inmediata:
- dolor repentino en el pecho;
- dolor en el pecho que se extiendo al brazo o al cuello;
- dificultades para respirar;
- hinchazón dolorosa y enrojecimiento en las piernas;
- coloración amarillenta de los ojos y la cara (ictericia);
- sangrado vaginal inesperado (sangrado intermenstrual) o manchado después de usar Lenzetto durante un tiempo o después de haber interrumpido el tratamiento;
- cambios en las mamas incluyendo hoyuelos en la piel, cambios en el pezón, bultos que pueda ver o notar;
- periodos menstruales dolorosos;
- mareo y desvanecimiento;
- cambios en el habla;
- cambios en la visión;
- dolores de cabeza de tipo migrañoso sin causa conocida.
Si alguno de los efectos adversos que sufre se agrava o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Se han notificado los siguientes efectos adversos con Lenzetto:
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
Dolor de cabeza, dolor abdominal, náuseas, erupción, prurito (picor), sangrado uterino irregular o sangrado vaginal incluyendo manchado, sensibilidad en las mamas, dolor en las mamas, aumento o pérdida de peso.
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
Reacciones de hipersensibilidad, estado de ánimo depresivo, insomnio (dificultad para dormir), mareo, vértigo (sensación de mareo o de "dar vueltas"), alteraciones visuales, palpitaciones (sentir los latidos del corazón), diarrea, dispepsia (indigestión), aumento de la presión arterial, eritema nodoso (caracterizado por nódulos cutáneos rojizos y dolorosos), urticaria (erupción o bultos generalizados o localizados), irritación de la piel, hinchazón debido a retención de líquidos (edema), dolor muscular, decoloración de las mamas, secreción mamaria, pólipos (pequeño crecimiento celular) en el útero o el cuello uterino, hiperplasia endometrial, quiste en el ovario, inflamación de los genitales (vaginitis), aumento de las enzimas hepáticas y del colesterol en sangre, dolor en las axilas.
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 personas)
Ansiedad, aumento o disminución del deseo sexual, migraña, intolerancia a las lentes de contacto, hinchazón abdominal, vómitos, aumento del vello corporal, acné, calambres musculares, menstruación dolorosa, síndrome premenstrual, aumento de tamaño de los senos, fatiga.
Se han notificado otros efectos adversoscon frecuencia "no conocida" (la frecuencia no pueden estimarse a partir de los datos disponibles) con Lenzetto durante el seguimiento post comercialización: pérdida de cabello (alopecia), cloasma (manchas color marrón dorado, llamadas "manchas del embarazo", especialmente en la cara), decoloración de la piel.
Los siguientes efectos adversos han sido notificados con otras THS:
Reacción alérgica grave que causa hinchazón de la cara o la garganta (angioedema), reacciones anafilactoides/anafilácticas (reacción alérgica grave que causa dificultad para respirar o mareos), intolerancia a la glucosa, depresión, alteraciones del estado de ánimo, irritabilidad, exacerbación de la corea (baile de San Vito), exacerbación de la epilepsia, demencia, exacerbación del asma, enfermedad de la vesícula biliar, coloración amarillenta de la piel (ictericia), inflamación del páncreas, neoplasia benigna del músculo liso del útero, diversos trastornos cutáneos: decoloración de la piel especialmente de la cara o cuello conocido como "manchas del embarazo" (cloasma); nódulos cutáneos rojizos y dolorosos (eritema nodoso); erupción con enrojecimiento en forma de diana o llagas (eritema multiforme), erupción hemorrágica, pérdida de cabello, dolor en las articulaciones, secreción de leche de las mamas, bultos en las mamas, aumento del tamaño de la neoplasia benigna del músculo liso del útero, cambios en la secreción y la capa interna del cérvix (cuello del útero), inflamación de la vagina, infecciones fúngicas en la vagina (candidiasis vaginal), niveles anormalmente bajos de calcio en sangre.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Lenzetto
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta después de CAD. La fecha de caducidad es el último día del mes que se indica.
Usar en los 56 días siguientes al primer uso.
No refrigerar o congelar este medicamento
No conservar a temperatura superior a 25 ºC.
Contiene etanol que es inflamable. Conservar alejado de calentadores, llamas abiertas y otras fuentes de ignición.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deLenzetto
- El principio activo es estradiol (como estradiol hemihidrato). Cada pulverización contiene 1,53 mg de estradiol (equivalente a 1,58 mg de estradiol hemihidrato).
- Los demás componentes son octisalato y etanol 96%.
Aspecto del producto y contenido del envase
Lenzetto es un aerosol transdérmico que contiene una solución de estradiol y octisalato en etanol. Provisto de una bomba dosificadora.
Lenzetto se presenta en un envase con tapa de plástico. En su interior hay un recipiente de vidrio que contiene 6,5 ml de solución y está diseñado para suministrar 56 pulverizaciones de 90 microlitros después del cebado de la bomba dosificadora. Marque cada pulverización hecha en la tabla de la caja.
Cada pulverización contiene 1,53 mg de estradiol.
Utilice solo el número de pulverizaciones indicado en la etiqueta de cada envase de Lenzetto, aunque el envase no esté completamente vacío.
Tamaños de los envase:
1 envase de 6,5 ml (56 pulverizaciones).
3 envases 3 x 6,5 ml (3 x 56 pulverizaciones).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungría
Responsable de la fabricación
Gedeon Richter România S.A.
Cuza Voda Street 99-105
Târgu-Mures
Rumanía - 540306
o
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungría
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Gedeon Richter Ibérica S.A.
Sabino Arana nº 28, 4º 2ª
08028 Barcelona, España
Fecha de la última revisión de este prospecto:Enero 2024
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia7.04 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LENZETTO 1,53 MG/DOSIS SOLUCION PARA PULVERIZACION TRANSDERMICAForma farmacéutica: GEL, 0,5 mgPrincipio activo: EstradiolFabricante: Orion CorporationRequiere recetaForma farmacéutica: GEL, 1 mgPrincipio activo: EstradiolFabricante: Orion CorporationRequiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 3 mgPrincipio activo: EstradiolFabricante: Merus Labs Luxco Ii S.À.R.L.Requiere receta
Médicos online para LENZETTO 1,53 MG/DOSIS SOLUCION PARA PULVERIZACION TRANSDERMICA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LENZETTO 1,53 MG/DOSIS SOLUCION PARA PULVERIZACION TRANSDERMICA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











