
KERIETTE DIARIO 0.1 MG/0.02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG

Cómo usar KERIETTE DIARIO 0.1 MG/0.02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
Keriette Diario 0,1 mg/0,02 mg comprimidos recubiertos con película EFG
Levonorgestrel / Etinilestradiol
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Keriette Diarioy para qué se utiliza
- Qué necesita saber antes de empezar a tomar Keriette Diario
- No use Keriette Diario
- Tenga especial cuidado con Keriette Diario
- Keriette Diarioy trombosis
- Keriette Diario ycáncer
- Sangrado entre los periodos
- Qué debe hacer si no hay sangrado en la semana de placebo
- Uso de otros medicamentos
- Pruebas analíticas
- Embarazo
- Lactancia
- Conducción y uso de máquinas
- Información importante sobre algunos de los componentes de Keriette Diario
- Cómo tomar Keriette Diario
- Cuándo puede comenzar con el primer blíster
- Si toma más Keriette Diariodel que debiera
- Si olvidó tomar Keriette Diario
- Qué debe hacer en caso de vómitos o diarrea intensa
- Retraso del periodo menstrual: qué debe saber
- Cambio del primer día de su periodo menstrual: qué debe saber
- Si desea dejar de tomar Keriette Diario
- Posibles efectos adversos
- Conservación de Keriette Diario
- Contenido del envase e información adicional
1. Qué es Keriette Diario y para qué se utiliza
- Keriette Diariouna píldora anticonceptiva que se utiliza para prevenir el embarazo.
- Cada comprimido de color rosa contiene una pequeña cantidad de dos hormonas femeninas diferentes, que se denominan levonorgestrel y etinilestradiol.
- Los comprimidos de color blanco no contienen principios activos y se conocen también como comprimidos de placebo.
Las píldoras anticonceptivas que contienen dos hormonas se conocen como “píldoras combinadas”.
2. Qué necesita saber antes de empezar a tomar Keriette Diario
Consideraciones generales
Antes de que pueda empezar a tomar Keriette Diario, su médico le hará algunas preguntas sobre sus antecedentes médicos y sobre sus familiares cercanos.También medirá su presión arterial y, dependiendo de su situación personal, también puede efectuar otras pruebas.
En este prospecto se describen varias situaciones en las que deberá dejar de usar Keriette Diario oen las cuales puede disminuir la fiabilidad de Keriette Diario.En estas situaciones, no deberá mantener relaciones sexuales o, en caso contrario, deberá adoptar otras precauciones anticonceptivas no hormonales, p. ej., usar un condón u otro método de barrera.No utilice los métodos del ritmo o de la temperatura.Esos métodos son poco fiables, ya que Keriette Diarioaltera los cambios mensuales de la temperatura corporal y del moco cervical.
Al igual que otros anticonceptivos hormonales, Keriette Diariono protege frente a la infección por el VIH (SIDA) o a cualquier otra enfermedad de transmisión sexual.
No useKeriette Diario
- Si tiene (o ha tenido en el pasado) un coágulo de sangre (trombosis) en un vaso sanguíneo de la pierna, los pulmones (embolia) u otros órganos
- Si tiene (o ha tenido en el pasado) un ataque cardiaco o un ictus
- Si tiene (o ha tenido en el pasado) una enfermedad que pueda predecir un ataque cardiaco (p. ej., angina de pecho, que provoca un dolor intenso en el pecho) o un ictus (p. ej., un pequeño ictus transitorio sin efectos residuales)
- Si tiene una enfermedad que pudiera aumentar el riesgo de trombosis en las arterias.Estas advertencias se aplican a las siguientes situaciones:
- diabetes con daños en los vasos sanguíneos
- presión arterial muy elevada
- concentraciones muy altas de grasa en sangre (colesterol o triglicéridos)
- Si tiene una alteración de la coagulación de la sangre (p. ej., deficiencia de proteína C)
- Si tiene (o ha tenido) una determinada forma de migraña (con los denominados síntomas focales neurológicos)
- Si tiene (o ha tenido) inflamación del páncreas (pancreatitis)
- Si tiene o ha tenido en el pasado una enfermedad hepática y si su función hepática aún no es normal
- Si padece o ha padecido un tumor en el hígado
- Si tiene (o ha tenido) o si hay sospecha de cáncer de mama o cáncer en órganos genitales
- Si tiene una hemorragia vaginal de causa desconocida
- Si no tiene el periodo desde hace varios meses sin una causa conocida
- Si es alérgica a levonorgestrel o etinilestradiol, o a cualquiera de los demás componentes de Keriette Diario. Esta alergia se puede reconocer por la aparición de prurito, erupción cutánea o inflamación.
- Si tiene hepatitis C y está tomando medicamentos que contienen ombitasvir / paritaprevir / ritonavir, dasabuvir, glecaprevir/pibrentasvir o sofosbuvir/velpatasvir/voxilaprevir (ver también la sección Uso de Keriette Diario con otros medicamentos).
Advertencias y precauciones
En algunas situaciones, tendrá que tomar precauciones especiales cuando use Keriette Diariocualquier otro anticonceptivo hormonal combinado, y a veces tendrá que acudir periódicamente a su médico. Si se encuentra en alguna de las situaciones siguientes, debe informar a su médico antes de comenzar a usar Keriette Diario. Además, tendrá que consultar con su médico si alguna de las siguientes situaciones aparece o empeora mientras usa Keriette Diario:
- Si un familiar cercano tiene o ha tenido cáncer de mama
- Si tiene una enfermedad del hígado o de la vesícula biliar
- Si sufre diabetes
- Si tiene depresión
- Si tiene enfermedad de Crohn o colitis ulcerosa (enfermedad intestinal inflamatoria)
- Si tiene SUH (síndrome urémico hemolítico), un trastorno de la sangre que provoca daños renales
- Si tiene anemia drepanocítica (una enfermedad hereditaria de los glóbulos rojos de la sangre)
- Si tiene epilepsia
- Si tiene LES (lupus eritematoso sistémico, un trastorno del sistema inmunitario)
- Si tiene una enfermedad que haya aparecido por primera vez durante el embarazo o durante el uso previo de hormonas sexuales (p. ej., pérdida de audición, porfiria [una enfermedad de la sangre], herpes gestacional [una erupción cutánea con vesículas que aparece durante el embarazo], corea de Sydenham [una enfermedad de los nervios en la cual se producen movimientos bruscos del cuerpo])
- Si tiene o ha tenido alguna vez cloasma (manchas pigmentadas de color marrón dorado que se conocen como “manchas del embarazo”, en especial en la cara). Si así fuera, evite la exposición directa a la luz solar o a la luz ultravioleta
- Si experimenta síntomas de angioedema como hinchazón de la cara, lengua y/o garganta, y/o dificultad para tragar o urticaria con posible dificultad para respirar, contacte con un médico de forma inmediata. Los productos que contienen estrógenos pueden causar o empeorar los síntomas del angioedema hereditario y adquirido.
Keriette Diario ytrombosis
Trombosis venosa
El uso de cualquier píldora combinada, incluido Keriette Diario, aumenta el riesgo de la mujer de desarrollar una trombosis venosa (formación de un coágulo de sangre en un vaso) en comparación con una mujer que no tome ninguna píldora anticonceptiva.
El riesgo de trombosis venosa aumenta en las usuarias de píldoras combinadas:
- Con la edad,
- Si hay sobrepeso,
- Si alguno de sus familiares ha tenido un coágulo de sangre (trombosis) en la pierna, el pulmón u otro órgano a temprana edad,
- Si debe someterse a una operación (cirugía), un periodo de inmovilización prolongado o si ha sufrido un accidente grave. Es importante que comente con su médico que está usando Keriette Diario, ya que podría tener que interrumpir el tratamiento. Su médico le dirá cuando tiene que empezar a tomar Keriette Diariootra vez. Normalmente, será en un plazo de dos semanas tras su recuperación.
Trombosis arterial
El uso de píldoras combinadas se ha relacionado con el aumento del riesgo de trombosis arteriales (obstrucción de una arteria), por ejemplo, en los vasos sanguíneos del corazón (ataque cardiaco) o del cerebro (ictus).
El riesgo de trombosis arterial aumenta en las usuarias de píldoras combinadas:
- Si fuma. Se le aconseja firmemente que deje de fumar cuando useKeriette Diario en especial si es mayor de 35 años
- Si tiene concentraciones elevadas de grasa en su sangre (colesterol o triglicéridos)
- Si tiene sobrepeso
- Si uno de sus familiares cercanos ha tenido un ataque cardiaco o un ictus a una edad temprana
- Si tiene la presión arterial alta
- Si tiene migrañas
- Si tiene problemas de corazón (un trastorno valvular o una alteración del ritmo cardiaco)
Deje de tomar Keriette Diarioy contacte inmediatamente con su médico si observa los signos posibles de trombosis, como:
- Dolor intenso o hinchazón de una de sus piernas
- Dolor intenso repentino en el pecho, que puede reflejarse hacia el brazo izquierdo
- Falta de aire de aparición repentina
- Tos repentina, sin una causa evidente
- Un dolor de cabeza inusual, intenso o de larga duración, o empeoramiento de una migraña
- Ceguera parcial o completa, o visión doble
- Dificultad o imposibilidad para hablar
- Vértigo o desvanecimiento
- Debilidad, sensación extraña o entumecimiento en cualquier parte del cuerpo
Keriette Diario ycáncer
Se han observado casos de cáncer de mama con una frecuencia ligeramente mayor en mujeres que toman píldoras anticonceptivas, pero se desconoce si se debe al tratamiento.Por ejemplo, pudiera ser que se detectasen más tumores en mujeres que toman píldoras combinadas porque son revisadas por su médico con mayor frecuencia. La aparición de tumores de mama ha sido gradualmente menor después de interrumpir el uso de los anticonceptivos hormonales combinados. Es importante revisar periódicamente sus mamas, y deberá contactar con su médico si notara algún bulto.
El cáncer de ovario se produce con menos frecuencia que el cáncer de mama. El uso de terapia hormonal sustitutiva (THS) con estrógenos solos o con combinación de estrógenos-progestágenos se ha asociado con un riesgo ligeramente mayor de cáncer de ovario.
El riesgo de cáncer de ovario varía con la edad. Por ejemplo, en mujeres de entre 50 y 54 años de edad que no siguen THS, se han observado alrededor de 2 casos de cáncer de ovario por cada 2.000 mujeres en un periodo de 5 años. En mujeres en tratamiento con THS durante 5 años, se han observado alrededor de 3 casos por cada 2.000 pacientes (es decir, alrededor de 1 caso adicional).
En casos raros, se han descrito tumores benignos de hígado y casos aún más raros de tumores malignos de hígado en las usuarias de píldoras anticonceptivas. Contacte con su médico si nota un dolor abdominal intenso inusual.
Sangrado entre los periodos
Durante los primeros meses de tratamiento con Keriette Diariopuede tener hemorragias inesperadas (sangrado fuera de la semana de placebo).Si esta hemorragia dura más de unos meses, o si comienza después de algunos meses, su médico debe investigar la causa.
Qué debe hacer si no hay sangrado en la semana de placebo
Si ha tomado correctamente todos los comprimidos activos de color rosa, no ha vomitado ni ha tenido diarrea intensa y no ha tomado otros medicamentos, es muy improbable que esté embarazada.
Si la hemorragia esperada no aparece en dos ocasiones sucesivas, podría estar embarazada.Contacte inmediatamente con su médico.No comience a tomar el siguiente blíster hasta que esté segura de que no está embarazada.
Keriette Diario y trastornos psiquiátricos
Algunas mujeres que utilizan anticonceptivos hormonales como Keriette Diario han notificado depresión o un estado de ánimo deprimido. La depresión puede ser grave y a veces puede inducir pensamientos suicidas. Si experimenta alteraciones del estado de ánimo y síntomas depresivos, póngase en contacto con su médico para obtener asesoramiento médico adicional lo antes posible.
Uso deKeriette Diario conotros medicamentos
Consulte siempre con el médico que le ha prescrito Keriette Diariosobre otros medicamentos o hierbas medicinales que ya esté utilizando.Además, comente con cualquier otro médico o dentista que le prescriba otros medicamentos (o con el farmacéutico que los dispense) que está usando Keriette Diario.Le podrán decir si necesita añadir otras medidas anticonceptivas (p. ej., condones) y, en ese caso, durante cuánto tiempo.
Algunos medicamentos disminuyen la eficacia de Keriette Diarioen la prevención del embarazo, o pueden provocar una hemorragia inesperada.
Entre ellos, se pueden citar los medicamentos usados para el tratamiento de la epilepsia (p. ej., primidona, fenitoína, barbitúricos, carbamazepina u oxcarbamazepina) y tuberculosis (p. ej., rifampicina) o infecciones por el VIH (ritonavir) u otras enfermedades infecciosas (como griseofulvina, ampicilina o tetraciclina), que aumenten la motilidad intestinal (como metoclopramida) y la planta medicinal hierba de San Juan.
Si desea usar hierbas medicinales que contengan hipérico mientras tome Keriette Diario, debe consultar primero a su médico.
Keriette Diariodisminuye la eficacia de otros medicamentos, como los que contienen ciclosporina, o el antiepiléptico lamotrigina (por lo que podría aumentar la frecuencia de las convulsiones).
No tome Keriette Diario si usted tiene hepatitis C y está tomando medicamentos que contienen ombitasvir / paritaprevir / ritonavir, dasabuvir, glecaprevir/pibrentasvir o sofosbuvir/velpatasvir/voxilaprevir, ya que estos medicamentos pueden producir aumentos en los resultados de pruebas hepáticas (aumento de la enzima hepática ALT).
Su médico le prescribirá otro tipo de anticonceptivo antes de comenzar el tratamiento con estos medicamentos.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Pruebas analíticas
Si necesita un análisis de sangre, avise a su médico o al personal del laboratorio que está tomando la píldora porque los anticonceptivos orales afectan a los resultados de algunas pruebas.
Embarazo
Si está embarazada, no debe tomar Keriette Diario. Si se queda embarazada mientras toma Keriette Diario, debe interrumpir inmediatamente su uso y contactar con su médico.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Lactancia
En general, no se recomienda usar Keriette Diariocuando la mujer está dando el pecho.Deberá consultar con su médico si desea tomar la píldora mientras está dando el pecho.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
No existe información que indique que el uso de Keriette Diarioafecte a la capacidad de conducir o usar máquinas.
Keriette Diariocontiene lactosa.Si su médico le ha indicado que padece cierta intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
3. Cómo tomar Keriette Diario
Si estuviera tomando otro anticonceptivo antes de comenzar a tomar Keriette Diario, ya sabrá que la mayoría de los anticonceptivos contienen 21 comprimidos.Con esos anticonceptivos, toma una píldora durante 21 días y después hay una semana en la que no toma ningún comprimido (intervalo sin medicamento).
El sistema a seguir cuando se usa Keriette Diarioes diferente.Después de tomar las 21 píldoras de color, debe continuar y tomar los 7 comprimidos de placebo, es decir, no hay un intervalo sin medicamento pero sí una semana de "placebo" (la semana en la que toma las píldoras de placebo de la fila 4).Como tiene que tomar un comprimido cada día y no hay un intervalo sin fármacos entre dos envases, tomar las píldoras se convertirá en algo rutinario y, en consecuencia, el riesgo de olvido de un comprimido es menor.
Las dos clases distintas de píldoras de color de Keriette Diarioestán colocadas en orden.Un envase contiene 28 comprimidos.
Tome un comprimido de Keriette Diariocada día, si es necesario con una pequeña cantidad de agua.Debe tomar los comprimidos cada día, más o menos a la misma hora.
No confunda los comprimidos:tome un comprimido de color rosa una vez al día en los primeros 21 días, y después un comprimido de color blanco cada día los últimos 7 días.Después, deberá comenzar un nuevo envase (21 comprimidos de color rosa y 7 comprimidos de color blanco).En consecuencia, no hay un intervalo sin toma de fármaco entre los envases.
El blíster contiene 28 comprimidos.La toma diaria durante 28 días consecutivos es importante para mantener el efecto anticonceptivo.
Junto a los blísteres se adjuntan unas etiquetas adhesivas con los días de la semana.Coja la etiqueta del día en que comience a tomar la píldora.Ponga la etiqueta en el blíster sobre las palabras “Pegar aquí la etiqueta con el día”.Cada día se alineará con una fila de píldoras.Es importante que tome su píldora cada día.Y tome el primer comprimido de la primera fila donde se indique la palabra “INICIO”.
Siga la dirección de la flecha que se indica en el blíster, tome primero los comprimidos de color rosa durante 21 días y después, los comprimidos de color blanco durante 7 días, hasta que haya tomado los 28 comprimidos.Después, deberá empezar a tomar el siguiente blíster.Esto significa que no hay un intervalo sin fármaco entre los envases.
Durante los 7 días de comprimidos blancos debería comenzar el sangrado (normalmente, el 2º o 3er día).Es lo que se conoce como hemorragia por privación y puede continuar cuando comience con el siguiente blíster.
Debe comenzar a tomar el siguiente blíster el mismo día de la semana, y la hemorragia por privación debería aparecer los mismos días cada mes.
Cuándo puede comenzar con el primer blíster
- Si no ha usado un anticonceptivo hormonal en el mes precedente.
Comience con Keriette Diarioel primer día del ciclo (que es el primer día de su menstruación). Si comienza a tomarKeriette Diarioen el primer día de su menstruación, se está protegiendo inmediatamente frente a un embarazo. También puede comenzar en los días 2-5 del ciclo pero, en ese caso, debe usar medidas extra de protección (p. ej. un condón) durante los primeros 7 días.
- Cambio desde otro anticonceptivo hormonal combinado o un anillo vaginal o un parche anticonceptivo combinado
Puede comenzar a tomar Keriette Diarioel día siguiente al periodo sin comprimidos de la píldora que acaba de terminar (o después del último comprimido inactivo de su píldora precedente).
Cuando cambie desde un anillo vaginal o un parche anticonceptivo combinado, siga los consejos de su médico.
- Cambio desde un método que contenga sólo progestágeno (píldora o inyección de progestágenos, implante o DIU liberadores de progestágenos)
Puede cambiar cualquier día desde la píldora que contiene sólo progestágenos (si usaba un implante o DIU, en el día de su retirada, y si recibía el progestágeno por vía inyectable, en la fecha en la que correspondería la siguiente inyección), pero en todos los casos debe aplicar medidas adicionales de protección (p. ej., un condón) durante los primeros 7 días en los que tome las nuevas píldoras.
- Después de un aborto o pérdida fetal
Siga las indicaciones de su médico.
- Después de tener un hijo
Después de tener un hijo,puede comenzar a tomar Keriette Diarioentre 21 y 28 días más tarde.Si comienza después del día 28, debe usar un método de barrera (p. ej., un condón) durante los primeros siete días de uso de Keriette Diario.
Si después de tener un hijo ha tenido relaciones antes de comenzar a tomar Keriette Diario(de nuevo), primero debe comprobar que no está embarazada o debe esperar hasta su siguiente hemorragia menstrual.
Pida consejo a su médico si no está segura de cuándo empezar.
- Si está dando el pecho y desea comenzar a tomar Keriette Diario (de nuevo) después de tener un hijo
Lea la sección sobre “Lactancia”.
Si toma másKeriette Diariodel que debe
No hay publicaciones sobre los resultados nocivos de tomar demasiados comprimidos deKeriette Diario. Si toma varios comprimidos de una vez, puede tener síntomas de náuseas y vómitos. Las niñas pequeñas pueden tener hemorragia vaginal.
Si ha tomado demasiados comprimidos deKeriette Diario, o si descubre que su hijo se ha tomado algunos, consulte con su médico o farmacéutico.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomarKeriette Diario
Los comprimidos de la cuartafila del envase son comprimidos de placebo. Si se olvidó de tomar una de esas pastillas, no afectará al efecto deKeriette Diario. Deberá tirar el comprimido de placebo olvidado para no prolongar la semana de placebo, lo que podría tener un efecto negativo en la efectividad de Keriette Diario.
Si se olvidó de tomar un comprimido de las filas 1, 2 o 3, deberá seguir las siguientes instrucciones:
- Si hace menos de 12 horasdel olvido del comprimido, la protección frente al embarazo no se altera. Todavía podrá tomar el comprimido en cuanto se acuerde y después tome los siguientes comprimidos a la hora habitual.
- Si hace más de 12 horasdel olvido del comprimido, la protección frente al embarazo puede disminuir. Cuantos más comprimidos se olvide de tomar, mayor es el riesgo de que se reduzca la protección frente al embarazo.
El riesgo de protección incompleta frente al embarazo es mayor si se olvida de tomar un comprimido al comienzo de un blíster (1ª fila) o al final de la semana 3 (3ª fila del blíster).
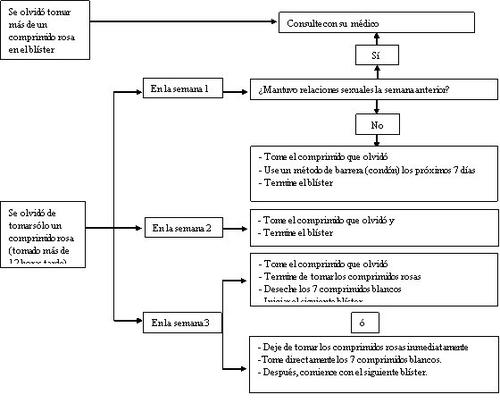
Por tanto, debe observar las reglas siguientes (ver también el diagrama, más adelante):
- Más de 1 comprimido olvidado del blíster
Consulte con su médico.
- Un comprimido olvidado en la semana 1
Tome el comprimido olvidado en cuanto se acuerde, incluso si ello quiere decir que tiene que tomar dos comprimidos al mismo tiempo.Tome después los comprimidos a la hora habitual y tome precauciones extraen los siguientes 7 días, por ejemplo, utilice condón.Si ha mantenido relaciones sexuales en la semana anterior al descuido, o si se olvidó de comenzar un nuevo blíster después del periodo sin comprimidos, debe darse cuenta de que hay riesgo de embarazo.En ese caso, consulte con su médico.
- Un comprimido olvidado en la semana 2
Tome el comprimido olvidado en cuanto se acuerde, incluso si ello quiere decir que tiene que tomar dos comprimidos al mismo tiempo.Tome los comprimidos después a la hora habitual.La protección frente al embarazo no se reduce y no tendrá que tomar precauciones extra.
- Un comprimido olvidado en la semana 3
Puede elegir entre 2 posibilidades:
- Tome el comprimido olvidado en cuanto se acuerde, incluso si ello quiere decir que tiene que tomar dos comprimidos al mismo tiempo.
En lugar de tomar los 7 comprimidos blancos de placebo, vaya directamente al siguiente blíster.
Lo más probable es que tenga una menstruación (hemorragia de privación) al terminar el segundo blíster pero también puede tener manchado o hemorragia intercurrente cuando tome el segundo blíster.
- También puede dejar de tomar los comprimidos activos, los de color rosa, y pasar a tomar directamente los 7 comprimidos de placebo, de color blanco (anote el día en el que se olvidó de tomar el comprimido).Después, continúe con el siguiente blíster.Si desea comenzar un nuevo blíster en un día concreto, tome los comprimidos de placebo durante menos de 7 días.
Si sigue una de esas dos recomendaciones, seguirá protegida frente al embarazo.
- Si se olvidó de tomar alguno de los comprimidos de un blíster y no tiene hemorragia en la semana del placebo, podría significar que está embarazada.Debe contactar con su médico antes de continuar con el siguiente blíster.
Qué debe hacer en caso de vómitos o diarrea intensa
Si vomita en las 3-4 primeras horas tras tomar el comprimido activo de color rosa o si tiene diarrea importante, existe el riesgo de que los principios activos del comprimido no se absorban por completo en su cuerpo.La situación es similar a cuando se olvida de tomar un comprimido.Después de vomitar o tener diarrea, debe tomar otro comprimido del blíster de reserva en cuanto sea posible.Si es posible, tómelo en las 12 horas siguientesal momento en que tomaría normalmente su comprimido.Si no es posible, o si ya han transcurrido 12 horas, debe seguir el consejo que se incluye en “Si olvidó tomarKeriette Diario”.
Retraso del periodo menstrual:qué debe saber
Aunque no sea lo recomendado, es posible retrasar su periodo menstrual (hemorragia de privación) hasta el final de un nuevo envase si no toma los comprimidos placebo de color blanco y comienza a tomar un segundo envase deKeriette Diario.Puede tener manchado (gotas o motas de sangre) o hemorragia intercurrente mientras usa el siguiente blíster.Después de los 7 días de tomar los comprimidos de placebo del segundo blíster, continúe con el siguiente blíster.
Es posible que tenga que pedir consejo a su médico antes de decidir si retrasa su ciclo menstrual.
Cambio del primer día de su periodo menstrual:qué debe saber
Si toma los comprimidos según las instrucciones, su periodo menstrual o hemorragia por privación comenzará en la semana de los comprimidos de placebo.Si tiene que cambiar este día, hágalo acortando el periodo de placebo, es decir, cuando tome los comprimidos blancos de placebo (pero nunca alargándolo).Por ejemplo, si su periodo de placebo comienza en un viernes y desea cambiar a un martes (3 días antes), debe comenzar un nuevo blíster 3 días antes de lo habitual.Si acorta mucho el intervalo de placebo (p. ej., 3 días o menos) es posible que no tenga ninguna hemorragia durante este periodo de placebo.Después, es posible que tenga manchado (gotas o motas de sangre) o hemorragia intercurrente.
Si no está segura de lo que tiene que hacer, pida consejo a su médico.
Si desea dejar de tomarKeriette Diario
Puede dejar de tomar Keriette Diariocuando lo desee. Si no desea quedarse embarazada, pida consejo a su médico sobre otros métodos fiables de control de natalidad.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede tener efectos adversos, aunque no todas las personas los sufran.
Informe siempre a su médico si presentara algún efecto secundario, en especial si este efecto secundario es intenso o persistente, o si observa algún cambio en su estado de salud que considere posiblemente debido a la píldora.
Se describen varios efectos secundarios relacionados con el uso de la píldora en las secciones “Keriette Diarioy trombosis” y “Keriette Diarioy cáncer”. Lea esos párrafos para obtener más información y consulte inmediatamente con su médico, si fuera necesario.
- Efectos secundarios frecuentes (pueden afectar a hasta a 1 de cada 10 mujeres): dolor de cabeza, cambios en el estado de ánimo (incluida la depresión), náuseas, dolor abdominal, mamas dolorosas, sensibilidad en las mamas, aumento de peso, erupción cutánea.
- Efectos secundarios poco frecuentes (pueden afectar a hasta a 1 de cada 100 mujeres): vómitos, diarrea, retención de líquidos o edema, migraña, pérdida del deseo sexual, aumento del tamaño de las mamas, urticaria.
- Efectos secundarios raros (pueden afectar a hasta a 1 de cada 1.000 mujeres): irritación ocular con el uso de lentes de contacto, hipersensibilidad, pérdida de peso, secreción mamaria, flujo vaginal, aumento de la libido, eritema nodoso (nódulos en las piernas), eritema multiforme (lesiones cutáneas).
Efectos adversos graves
Contacte con un médico de forma inmediata si experimenta cualquiera de los siguientes síntomas de angioedema: hinchazón de la cara, lengua y/o garganta, y/o dificultad para tragar o urticaria con posible dificultad para respirar (ver también sección “Advertencias y precauciones”).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte con su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de uso humano, website: www.notificaRAM.es. Mediante la comunicación de efectos adversos puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Keriette Diario
Mantener fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 30°C.
No utilice Keriette Diariodespués de la fecha de caducidad que aparece en el envase exterior y en el blíster después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de su farmacia habitual. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deKeriette Diario
Los principios activos son levonorgestrel y etinilestradiol.
Keriette Diariocomprimidos recubiertos con película contiene comprimidos de 2 colores:
- Cadacomprimido de color rosa contiene 0,1 mg de levonorgestrel y 0,02 mg de etinilestradiol.Los demás componentes (excipientes) son lactosa anhidra, povidona K-30 (E1201), estearato de magnesio (E572) y opadry II rosa [alcohol polivinílico, talco (E553b), dióxido de titanio (E171), polietilenglicol 3350, laca de aluminio roja (E129), lecitina (E322), óxido de hierro rojo (E172) y laca de aluminio azul (E1329)].
- Cada comprimido de color blanco (comprimidos inactivos o comprimidos de placebo) contiene sólo excipientes (sin principios activos), que son lactosa anhidra, povidona K-30 (E1201), estearato de magnesio (E572) y opadry II blanco [alcohol polivinílico, talco (E553b), dióxido de titanio (E171), polietilenglicol 3350].
Aspecto del producto y contenido del envase
- Cada comprimido activo recubierto con película es redondo de color rosa.
- Cada comprimido de placebo recubierto con película es redondo de color blanco.
- Keriette Diariose comercializa en blísteres de 28 comprimidos: 21 comprimidos activos de color rosa y 7 comprimidos de placebo de color blanco.
- Los tamaños del envase son de 1, 3 o 6 blísteres, y cada blíster contiene 28 comprimidos. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Kern Pharma, S.L.
C/Venus 72, Pol. Ind. Colón II
08228 Terrassa (Barcelona)
España
Responsable de la fabricación
Laboratorios León Farma, S.A.
Pol. Ind. Navatejera;
C/La Vallina s/n;
24193 - Villaquilambre, León
España
Fecha de la última revisión de este prospecto: Noviembre 2022
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia2.5 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KERIETTE DIARIO 0.1 MG/0.02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFGForma farmacéutica: COMPRIMIDO, 0,1 mg/0,02 mgPrincipio activo: levonorgestrel and ethinylestradiolFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 0,1 mg/0,02 mgPrincipio activo: levonorgestrel and ethinylestradiolFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: COMPRIMIDO, 0,10 mg/0,02 mgPrincipio activo: levonorgestrel and ethinylestradiolFabricante: Sandoz Farmaceutica S.A.Requiere receta
Médicos online para KERIETTE DIARIO 0.1 MG/0.02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KERIETTE DIARIO 0.1 MG/0.02 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






