GONAPEPTYL DEPOT 3.75 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION

How to use GONAPEPTYL DEPOT 3.75 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What GONAPEPTYL DEPOT is and what it is used for
- GONAPEPTYL Depot contains triptorelin (as triptorelin acetate). Triptorelin belongs to a group of medicines called GnRH analogues. One of its actions is to decrease the production of sex hormones in the body.
- Before using GONAPEPTYL DEPOT
- How to use GONAPEPTYL DEPOT
- Possible Adverse Effects
- Storage of GONAPEPTYL DEPOT
- Additional Information
Introduction
Package Leaflet: Information for the User
GONAPEPTYL DEPOT 3.75 milligrams
Powder and solvent for injectable suspension
Triptorelin
Read all of this leaflet carefully before you start using this medicine
- Keep this leaflet, you may need to read it again. If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the pack
- What GONAPEPTYL DEPOT is and what it is used for
- Before you start using GONAPEPTYL DEPOT
- How to use GONAPEPTYL DEPOT
- Possible side effects
- Storing GONAPEPTYL DEPOT
- Further information
1. What GONAPEPTYL DEPOT is and what it is used for
GONAPEPTYL Depot contains triptorelin (as triptorelin acetate). Triptorelin belongs to a group of medicines called GnRH analogues. One of its actions is to decrease the production of sex hormones in the body.
It is used:
In men:
- Treatment of locally advanced or metastatic hormone-dependent prostate cancer.
In women:
To suppress ovarian hormone levels for:
- Reducing the size of uterine fibroids (non-cancerous tumors that grow from the muscle layer of the uterus).
- Treating endometriosis (the presence of uterine tissue outside the uterus).
In children:
- Treatment of central precocious puberty (puberty that occurs prematurely but with normal physical and hormonal changes of puberty).
2. Before using GONAPEPTYL DEPOT
You must not use GONAPEPTYL Depot:
- If you are allergic to triptorelin or any of the other ingredients of GONAPEPTYL Depot.
- If you are allergic to gonadotropin-releasing hormone (GnRH) or any other GnRH analogue.
In women:
- If you are pregnant or breastfeeding.
Be cautious when using GONAPEPTYL Depot
In men and women:
- Depression has been reported in patients treated with Gonapeptyl, which can be severe. If you are taking Gonapeptyl and develop depressive mood, inform your doctor.
- GONAPEPTYL Depot may cause changes in mood.
- Treatment with GONAPEPTYL Depot may rarely cause bleeding in the brain (pituitary apoplexy). Inform your doctor immediately if you have sudden severe headache, vomiting, or vision disturbances.
- Treatment with GONAPEPTYL Depot may cause bone thinning, which increases the risk of bone fractures.
- If you have an increased risk of bone thinning (osteoporosis), you should inform your doctor before using GONAPEPTYL Depot. Risk factors include:
- If any of your close relatives have osteoporosis.
- If you drink excessive amounts of alcohol, have a poor diet, and/or smoke heavily.
- If you are also being treated with certain medications that may affect bone strength.
In men:
Tell your doctor:
During treatment:
During the start of treatment with GONAPEPTYL Depot, you may experience worsening of your disease symptoms.
Consult your doctor if any of your disease symptoms worsen.
In women:
Tell your doctor:
During treatment:
Non-hormonal contraceptive measures, such as condoms or diaphragms, should be used during the first month after the first injection. They should also be used from week 4 after the last injection until your periods (menstruation) resume.
Your menstruation will stop during treatment. Once treatment is stopped, your periods (menstruation) will resume 7-12 weeks after the final injection.
If your periods (menstruation) continue during treatment, please inform your doctor.
In children:
- Treatment should only be started in girls under 9 years old and boys under 10 years old.
Tell your doctor:
During treatment:
During the first month of treatment, girls may experience mild to moderate vaginal bleeding episodes.
Once treatment is stopped, the development of puberty characteristics will occur. In most girls, menstruation will occur within a year after stopping treatment, and in most cases, it will be regular.
For any possible side effects, please see section 4.
Using other medicines
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription.
GONAPEPTYL Depot may interfere with some medicines used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with other medications (e.g., methadone (used for pain relief and drug addiction detoxification), moxifloxacin (an antibiotic), antipsychotics used for severe mental illnesses).
Driving and using machines
No effects on the ability to drive and use machines are known.
However, it cannot be excluded that the ability to drive or use machines may be affected during treatment due to some of the side effects (dizziness, sleep disturbances/insomnia, and eye vision disturbances). Take extra precautions if you experience these side effects.
Pregnancy and breastfeeding
No effects on the ability to drive and use machines are known.
However, it cannot be excluded that the ability to drive or use machines may be affected during treatment due to some of the side effects (dizziness, sleep disturbances/insomnia, and eye vision disturbances). Take extra precautions if you experience these side effects.
Gonapeptyl should not be used during pregnancy and breastfeeding (see the section “You must not use GONAPEPTYL Depot”). If you think you may be pregnant, your doctor should rule out pregnancy before using Gonapeptyl Depot.
Fertile women should use non-hormonal effective contraceptive methods, such as condoms or diaphragms, during treatment with Gonapeptyl Depot until menstruation resumes.
Driving and using machines
No effects on the ability to drive and use machines are known.
However, it cannot be excluded that the ability to drive or use machines may be affected during treatment due to some of the side effects (dizziness, sleep disturbances/insomnia, and eye vision disturbances). Take extra precautions if you experience these side effects.
Gonapeptyl should not be used during pregnancy and breastfeeding (see the section “You must not use GONAPEPTYL Depot”). If you think you may be pregnant, your doctor should rule out pregnancy before using Gonapeptyl Depot.
Fertile women should use non-hormonal effective contraceptive methods, such as condoms or diaphragms, during treatment with Gonapeptyl Depot until menstruation resumes.
Driving and using machines
No effects on the ability to drive and use machines are known.
However, it cannot be excluded that the ability to drive or use machines may be affected during treatment due to some of the side effects (dizziness, sleep disturbances/insomnia, and eye vision disturbances). Take extra precautions if you experience these side effects.
Gonapeptyl should not be used during pregnancy and breastfeeding (see the section “You must not use GONAPEPTYL Depot”). If you think you may be pregnant, your doctor should rule out pregnancy before using Gonapeptyl Depot.
Fertile women should use non-hormonal effective contraceptive methods, such as condoms or diaphragms, during treatment with Gonapeptyl Depot until menstruation resumes.
Driving and using machines
No effects on the ability to drive and use machines are known.
However, it cannot be excluded that the ability to drive or use machines may be affected during treatment due to some of the side effects (dizziness, sleep disturbances/insomnia, and eye vision disturbances). Take extra precautions if you experience these side effects.
Gonapeptyl should not be used during pregnancy and breastfeeding (see the section “You must not use GONAPEPTYL Depot”). If you think you may be pregnant, your doctor should rule out pregnancy before using Gonapeptyl Depot.
Fertile women should use non-hormonal effective contraceptive methods, such as condoms or diaphragms, during treatment with Gonapeptyl Depot until menstruation resumes.
3. How to use GONAPEPTYL DEPOT
The powder and solvent are mixed and injected, usually by healthcare professionals.
Depending on the indication for your treatment, the appropriate dose will be administered by injection into a muscle (intramuscular) or under the skin (subcutaneous).
In men:
- GONAPEPTYL Depot is usually administered as an injection every 4 weeks as long-term treatment.
In women:
- GONAPEPTYL Depot is usually administered as an injection every 4 weeks for a maximum of 6 months.
- Treatment should be started during the first five days of the menstrual cycle.
In children:
- At the start of treatment, an injection of triptorelin should be administered on days 0, 14, and 28.
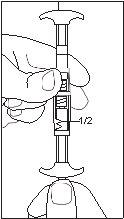
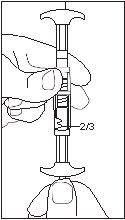
- The dose is adjusted according to the child's weight. Children weighing less than 20 kilograms are administered 1.875 milligrams (1/2 dose), children weighing 20-30 kilograms are administered 2.5 milligrams (2/3 dose), and children weighing more than 30 kilograms are administered 3.75 milligrams.
- Subsequent injections are administered every 3-4 weeks, depending on the effect.
The duration of treatment should be supervised by your doctor.
If you use more GONAPEPTYL DEPOT than you should:
It is unlikely that you will be given more GONAPEPTYL Depot than you should have received. If more GONAPEPTYL Depot has been administered than it should have been, inform your doctor or pharmacist immediately.
If you stop using GONAPEPTYL DEPOT
Treatment with GONAPEPTYL Depot should only be stopped under the advice of your doctor. If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, GONAPEPTYL Depot can cause adverse effects, although not all people suffer from them.
General (all patients):
If you experience swelling of the face, lips, mouth, or throat that may cause difficulty swallowing or breathing, consult your doctor or go to the nearest health service.
There have been reports of enlargement of existing pituitary tumors during treatment with LH-RH agonists, but these have not been observed with triptorelin treatment.
In Men:
Due to the increase in testosterone levels at the start of treatment, symptoms for which you are being treated (i.e., urinary obstruction, back pain, spinal cord compression, muscle weakness, and edema in the legs and weakness and tingling in the feet and hands) may worsen initially.
Very common, more than 1 patient in 10 treated:most of the adverse effects of GONAPEPTYL Depot in men occur due to the decrease in testosterone levels. Impotence, decreased libido, hot flashes, bone pain, difficulty and pain when urinating may occur.
Common, between 1 and 10 patients in 100 treated:allergic reaction, depressive mood, mood changes, depression, sleep disorders, nausea, muscle and joint pain, fatigue, reaction at the injection site, pain at the injection site, irritability, excessive sweating, headache, and increased breast volume in men.
Uncommon, between 1 and 10 patients in 1000 treated:elevation of some liver enzymes, anaphylactic reaction, testicular atrophy, high blood pressure, decreased appetite, dry mouth, upper abdominal pain, worsening of asthma, weight changes, embolism, hair loss, and reduced hair growth.
Frequency not known, cannot be estimated from available data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In Women:
Very common, more than 1 patient in 10 treated:decreased libido, mood changes, sleep disorders, hot flashes, abdominal pain, bone pain, excessive sweating, vaginal bleeding/staining, vulvovaginal dryness, pain during sexual intercourse, painful menstruation, increased ovarian size, pelvic pain, weakness, and headache.
Common, between 1 and 10 patients in 100 treated:allergic reaction, depressive mood, depression, nausea, muscle and joint pain, fatigue, reaction at the injection site, pain at the injection site, irritability.
Uncommon, between 1 and 10 patients in 1000 treated:anaphylactic reaction, visual disturbances, feeling of tingling, numbness, or prickling, back pain, increased cholesterol in the blood, elevation of some liver enzymes.
Frequency not known, cannot be estimated from available data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In Children:
Common, between 1 and 10 patients in 100 treated:mood changes, depression.
Uncommon, between 1 and 10 patients in 1000 treated:in girls, vaginal bleeding or discharge may occur. Nausea, vomiting, and anaphylactic reaction have been seen.
Frequency not known, cannot be estimated from available data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of GONAPEPTYL DEPOT
Keep out of the reach and sight of children.
Do not use GONAPEPTYL Depot after the expiration date shown on the packaging. The expiration date is the last day of the month indicated.
Store in the refrigerator (between 2°C and 8°C). Store in the original packaging.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Additional Information
Composition of GONAPEPTYL DEPOT
- In each preloaded syringe, the powder contains 4.12 milligrams of triptorelin acetate, equivalent to 3.75 milligrams of the active ingredient, triptorelin.
- The other components are: Poly-(d,l lactide coglycolide), dicaprylate propylene glycol.
The solvent contains:
- Dextran 70, polysorbate 80, sodium chloride, sodium dihydrogen phosphate dihydrate, sodium hydroxide, and water for injection.
This product contains less than 1 millimole of sodium (3.69 milligrams/milliliter or 0.160 millimoles/milliliter) per dose, i.e., it is essentially "sodium-free".
Appearance of Gonapeptyl Depot and packaging content
It is presented in packaging of 1 set of: 1 or 3 pairs of preloaded syringes (powder and solvent).
Not all presentations are available in Spain.
Marketing authorization holder and manufacturer:
Marketing authorization holder:
FERRING S.A.U.
C/ del Arquitecto Sánchez Arcas 3, 1º
28040 Madrid. Spain
Manufacturer:
FERRING GmbH
Wittland 11,
D-24109 Kiel
Germany.
This medicine is authorized in the Member States of the European Economic Area under the following names:
GONAPEPTYL DEPOT (Belgium, Greece, Italy, Luxembourg, Netherlands, Sweden, Spain, Portugal, United Kingdom), GONAPEPTYL 3.75 mg (France), GONAPEPTYL DEPOT 3.75 mg (Ireland), DECAPEPTYL DEPOT (Czech Republic, Denmark, Iceland, Estonia, Germany, Latvia, Lithuania, Norway, Poland, Slovakia), DECAPEPTYL DEPOT 3.75 (Finland), DECAPEPTYL N (Germany), UROPEPTYL DEPOT (Germany), DECAPEPTYL GYN (Germany), GYNOPEPTYL (Germany), DECAPEPTYL CR 3.75 (Netherlands), DECAPEPTYL DEPOT-Retardmikrokapseln und Suspensionsmittel für Einmalspritzen (Austria), DECAPEPTYL DEPOT injection (Hungary), DECAPEPTYL Retard injectionspräparat i.m/s.c (Switzerland).
This prospectus was approved in March 2023.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
The following information is intended only for healthcare professionals:
INSTRUCTIONS FOR USE
Important information:
- Store Gonapeptyl Depot in the packaging in the refrigerator.
- Make sure to inject Gonapeptyl Depot within 3 minutes after reconstitution.
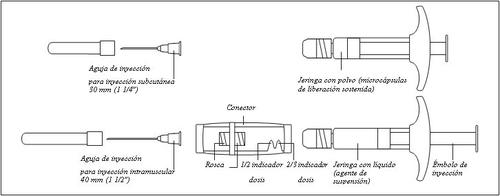 General description of the components of Gonapeptyl Depot:
General description of the components of Gonapeptyl Depot:
- Preparation
To ensure the correct preparation of the suspension, the following instructions must be strictly followed:
A | B | C | |||
|
|
| |||
Make sure not to touch the connector threads | Make sure not to push the injection plunger. | Always connect the syringe with powder to the connector before connecting the syringe with liquid. |
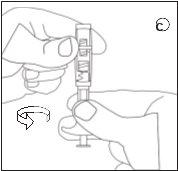
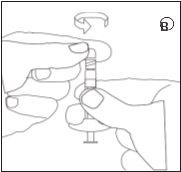
D | E | ||||
|
| CONTINUED ON NEXT PAGE TURN PAGE | |||
Make sure not to push the injection plunger. |
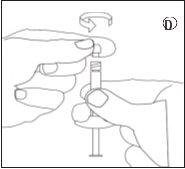
- Reconstitution
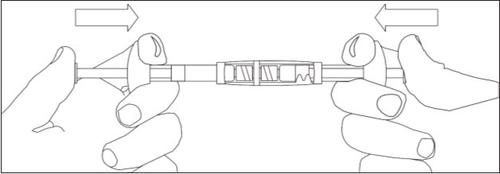
To mix the suspension:
- Inject all the liquid into the syringe with powder.
- Slowly push the suspension back and forth in the two syringes until the suspension is a homogeneous white or slightly yellowish color. Be careful to keep the syringes straight; do not tilt them.
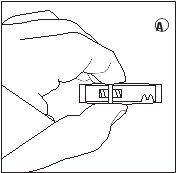
1/2 or 2/3 dose for children:Use the dose indicators on the connector to measure 1/2 or 2/3 of the dose: • Make sure the suspension is in the syringe connected to the side of the connector without dose indicators. • Turn the syringes to a vertical position with the syringe containing the suspension at the top. • Wait a few seconds to allow the foam to separate. • Slowly pull the injection plunger of the empty syringe downwards until the suspension reaches the 1/2 or 2/3 indicator. |
1/2 DOSE |
2/3 DOSE |
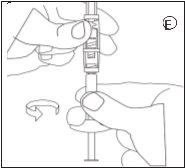
3.Injection
- Unscrew the syringe with the ready-to-inject suspension from the connector.
- Screw the injection needle into the syringe.
- Inject the suspension within 3 minutes.

Gonapeptyl Depot is for single use only, and any unused suspension should be discarded.
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GONAPEPTYL DEPOT 3.75 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTIONDosage form: INJECTABLE, 0.1 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription requiredDosage form: INJECTABLE, 3.75 mg triptorelin/vialActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription requiredDosage form: INJECTABLE, 22.5 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.U.Prescription required
Online doctors for GONAPEPTYL DEPOT 3.75 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION
Discuss questions about GONAPEPTYL DEPOT 3.75 mg POWDER AND SOLVENT FOR SUSPENSION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions