ESTRADOT 75 microgramos/24 HORAS, PARCHE TRANSDERMICO


Cómo usar ESTRADOT 75 microgramos/24 HORAS, PARCHE TRANSDERMICO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: informacion para el usuario
Estradot 75 microgramos/24 horas parche transdérmico
Estradiol (como hemihidrato)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Estradot y para qué se utiliza
- Qué necesita saber antes de empezar a usar Estradot
- Cómo usar Estradot
- Posibles efectos adversos
- Conservación de Estradot
- Contenido del envase e información adicional
1. Qué es Estradot y para qué se utiliza
Estradot es un Tratamiento Hormonal de Sustitución (THS) que contiene la hormona femenina de tipo estrógeno.
Estradot se utiliza en mujeres postmenopáusicas cuando han transcurrido al menos 12 meses desde su último periodo natural.
Estradot se presenta como un parche que se aplica en la piel.
Estradot se utiliza para:
Alivio de los síntomas aparecen en la menopausia
Durante la menopausia, la cantidad de estrógenos producidos por el cuerpo de la mujer disminuyen. Esto puede causar síntomas como oleadas repentinas de calor en la cara, cuello y pecho (sofocos). Estradot alivia estos síntomas tras la menopausia. Sólo se le recetará Estradot si sus síntomas dificultan seriamente su vida cotidiana.
Prevención de osteoporosis
Después de la menopausia, algunas mujeres pueden desarrollar fragilidad de los huesos (osteoporosis). Consulte con su médico sobre todas las opciones de tratamiento disponibles. Si tiene un riesgo elevado de sufrir fracturas debido a la osteoporosis y otros medicamentos no son adecuados para usted, puede usar Estradot para prevenir la osteoporosis después de la menopausia.
2. Qué necesita saber antes de empezar a usar Estradot
Historia médica y revisiones regulares
El uso de THS conlleva riesgos que se deben considerar al decidir su uso o si se continúa el tratamiento.
La experiencia en el tratamiento de mujeres con menopausia prematura (debido a fallo ovárico o cirugía) es limitada. Si usted tiene menopausia prematura, los riesgos de usar THS pueden ser diferentes. Por favor, consulte a su médico.
Antes de empezar (o retomar) la THS, su médico le preguntará por su historia médica personal y familiar. Su médico podrá decidir llevar a cabo una exploración física. Ésta podrá incluir un examen de sus pechos y/o un examen interno, si fuese necesario.
Una vez empezado el tratamiento con Estradot, debe visitar a su médico para realizar revisiones regulares (por lo menos una vez al año). En estas revisiones, hable con su médico sobre los beneficios y riesgos de continuar con Estradot.
Realícese revisiones de mama periódicas, tal y como recomiende su médico.
No useEstradot
si alguno de los siguientes casos le afectan a usted. Si no está segura de alguno de los puntos aquí descritos, consulte a su médicoantes de usar Estradot.
No use Estradot:
- si padece o ha padecido cáncer de mama, o si hay sospecha de que pueda tenerlo;
- si tiene un cáncer dependiente de estrógenos, tal como cáncer de la pared interna del útero (endometrio), o si hay sospecha de que pueda tenerlo;
- si presenta hemorragia vaginalanormal;
- si tiene un engrosamiento excesivo de la pared interna del útero.(hiperplasia de endometrio) para el cual no esté recibiendo tratamiento;
- si padece o ha padecido la formación de un coágulo de sangre en una vena(trombosis), como por ejemplo en las piernas (trombosis venosa profunda) o en el pulmón (embolismo pulmonar);
- si presenta un trastorno de la coagulación sanguínea(como deficiencia de proteína C, de proteína S o de antitrombina);
- si padece o ha padecido recientemente una enfermedad causada por coágulos sanguíneos en las arterias, como un ataque al corazón,un accidente cerebrovascularo una anginade pecho;
- si padece o ha padecido una enfermedad del hígadoy sus pruebas de función hepática no se han normalizado;
- si presenta una enfermedad rara de la sangre denominada porfiriaque se transmite de padres a hijos (hereditario);
- si es alérgico(hipersensible) a estradiol, o a alguno de los demás componentes de este medicamento (incluidos en la sección 6 Contenido del envase e información adicional);
Si durante el uso de Estradot experimenta por primera vez alguna de las condiciones anteriormente mencionadas, interrumpa el tratamiento enseguida y consulte con su médico inmediatamente.
Advertencias y precauciones
Informe a su médico si sufre o ha sufrido alguna de las siguientes situaciones antes de comenzar el tratamiento, pues pueden aparecer de nuevo o empeorar durante el tratamiento con Estradot. En ese caso, debería acudir a su médico con mayor frecuencia para revisiones periódicas:
- fibromas dentro del útero;
- crecimiento de la pared interna del útero fuera del útero (endometriosis) o antecedentes de crecimiento excesivo de la pared interna del útero (hiperplasia endometrial);
- aumento del riesgo de desarrollar coágulos de sangre (ver “Coágulos de sangre en una vena (trombosis)”;
- aumento del riesgo de contraer un cáncer que dependa de la acción de estrógenos (como por ejemplo, cuando su madre, hermana o abuela han padecido cáncer de mama);
- aumento de la tensión arterial;
- trastorno del hígado, como por ejemplo un tumor benigno en el hígado;
- diabetes;
- piedras en la vesícula biliar (cálculos biliares);
- migraña o dolor de cabeza graves;
- una enfermedad del sistema inmunitario que afecta a varios órganos del cuerpo (lupus eritematoso sistémico, LES);
- epilepsia;
- asma;
- una enfermedad que afecte al tímpano y al oído (otosclerosis);
- un nivel muy elevado de grasa en su sangre (triglicéridos);
- retención de líquidos debida a problemas cardiacos o renales;
- angioedema hereditario y adquirido.
Interrumpa el uso de Estradot y acuda a un médico inmediatamente.
Si sufre alguna de las siguientes situaciones mientras usa THS:
- cualquiera de las condiciones mencionadas en la sección ‘No use Estradot’
- coloración amarillenta de la piel o en el blanco de los ojos (ictericia). Esto puede ser un signo de enfermedad hepática
- hinchazón de la cara, lengua o garganta y dificultad para tragar o urticaria acompañados de dificultad para respirar, que sugieren un angioedema;
- aumento significativo de su tensión arterial (los síntomas pueden ser dolor de cabeza, cansancio, mareos)
- dolores de cabeza de tipo migrañoso que ocurren por primera vez
- si se queda embarazada
- si aprecia signos de un coágulo sanguíneo, como:
- hinchazón dolorosa y enrojecimiento de las piernas;
- dolor de pecho repentino;
- dificultad para respirar.
Para más información, ver ‘Coágulos de sangre en una vena (trombosis)’.
Nota: Estradot no es un anticonceptivo. En el caso de que hayan transcurrido menos de 12 meses desde su última menstruación o tenga menos de 50 años, aún puede necesitar medidas anticonceptivas adicionales para prevenir un embarazo. Hable con su médico para solicitar consejo.
THS y cáncer
Engrosamiento de la pared interna del útero (hiperplasia de endometrio) y cáncer de la pared interna del útero (endometrio)
La toma de THS con productos de estrógenos solos aumentará el riesgo de padecer engrosamiento de la pared interna del útero (hiperplasia endometrial) y de cáncer de la pared interna del útero (cáncer de endometrio).
La adición de un progestágeno al tratamiento con estrógeno durante por lo menos 12 días de cada ciclo de 28 días la protege de este riesgo adicional. Por lo tanto, su médico le recetará un progestágeno por separado si todavía conserva su útero. Si le han extirpado el útero (histerectomía), pregunte a su médico acerca de si puede tratarse con este medicamento con seguridad sin el uso de un progestágeno.
En mujeres de edades comprendidas entre los 50 y 65 años, que conservan el útero y no están siendo tratadas con THS, una media de 5 de cada 1000 serán diagnosticadas de cáncer de la pared interna del útero.
En el caso de mujeres de edades comprendidas entre los 50 y 65 años, con útero y en tratamiento de THS con estrógenos solos, entre 10 y 60 mujeres de cada 1000 serán diagnosticadas de cáncer de endometrio (esto es, entre 5 y 55 casos adicionales), dependiendo de la dosis y de la duración de la terapia.
Hemorragias no esperadas
Experimentará un sangrado una vez al mes (denominado sangrado por menstruación) mientras use Estradot en combinación con un progestágeno. Pero, si sufre hemorragias o manchados de sangre no esperados a parte de su sangrado mensual, que:
- continúan durante más de los 6 primeros meses;
- comienzan después de que haya estado usando Estradot durante más de 6 meses;
- continúan después de que haya interrumpido el uso de Estradot;
acuda a su médico lo antes posible.
Cáncer de mama
La evidencia muestra que el uso de terapia hormonal sustitutiva (THS) con combinación de estrógeno-progestágeno o con estrógenos solo, aumenta el riesgo de cáncer de mama. El riesgo adicional depende del tiempo durante el que se use la THS. El riesgo adicional se hace evidente después de 3 años de uso. Tras suspender la THS, el riesgo adicional disminuirá con el tiempo, pero el riesgo puede persistir durante 10 años o más si ha usado THS durante más de 5 años.
Comparación
En mujeres de 50 a 54 años que no estén utilizando THS, en una media de 13 a 17 de cada 1000 serán diagnosticadas con cáncer de mama en un periodo de 5 años.
En mujeres de 50 años que inicien una terapia hormonal sustitutiva con solo estrógenos para 5 años, habrá entre 16 y 17 casos por cada 1000 mujeres usuarias (es decir, entre 0 y 3 casos adicionales).
En mujeres de 50 años que inicien tratamiento con THS combinada estrógeno-progestágeno durante 5 años, aparecerán de 21 casos por cada 1000 mujeres usuarias (es decir, entre 4 y 8 casos).
En mujeres de 50 a 59 años que no estén tomando THS, se diagnosticarán un promedio de 27 casos de cáncer de mama por cada 1000 mujeres en un periodo de 10 años.
En mujeres de 50 años que inicien una terapia hormonal sustitutiva solo con estrógenos durante 10 años, habrá 34 casos por cada 1000 mujeres usuarias (es decir, siete casos adicionales).
En mujeres de 50 años que inicien una THS con estrógenos –progestágenos durante 10 años, habrá 48 casos de cada 1000 usuarias (es decir, 21 casos adicionales).
- Examine sus pechos regularmente. Acuda a su médico si detecta cualquier cambio como, por ejemplo:
- surcos o hendiduras la piel;
- cambios en los pezones;
- cualquier bulto que pueda ver o notar.
Además, se le recomienda participar en programas de cribado mediante mamografía. En las mamografías de cribado es importante que informe al enfermero o al profesional sanitario, que está realizando los Rayos X, que está usando THS, ya que esta medicación puede aumentar la densidad de sus pechos, lo que puede afectar al resultado de la mamografía. Cuándo aumenta la densidad del pecho, la mamografía puede no detectar todos los bultos.
Cáncer de ovario
El cáncer de ovario se produce con menos frecuencia que el cáncer de mama. El uso de THS con estrógenos solos o con combinación de estrógenos-progestágenos se ha asociado con un riesgo ligeramente mayor de cáncer de ovario.
El riesgo de cáncer de ovario varía con la edad. Por ejemplo, en mujeres de entre 50 y 54 años de edad que no siguen THS, se han observado alrededor de 2 casos de cáncer de ovario por cada 2000 mujeres en un período de 5 años. En mujeres en tratamiento con THS durante 5 años, se han observado alrededor de 3 casos por cada 2000 pacientes (es decir, alrededor de 1 caso adicional).
Efecto de la THS sobre el corazón y la circulación
Coágulos de sangre en una vena (trombosis)
El riesgo de coágulos de sangre en las venases aproximadamente de 1.3 a 3 veces mayor para las usuarias de THS que para las no usuarias, especialmente durante el primer año de tratamiento.
Los coágulos de sangre pueden ser graves, y si alguno se desplaza a los pulmones, puede provocar dolor de pecho, dificultad para respirar, desvanecimiento o incluso la muerte.
Tiene más probabilidades de desarrollar un coágulo sanguíneo en sus venas con la edad y si experimenta alguna de las siguientes situaciones. Informe a su médico si alguna de las situaciones siguientes le ocurre a usted:
- no puede caminar por un periodo de tiempo prolongado debido a cirugía mayor, lesión o enfermedad (ver también la sección 3, Si necesita cirugía);
- tiene sobrepeso considerable (IMC>30 kg/m2);
- tiene un problema de coagulación de la sangre que necesita tratamiento a largo plazo con un medicamento usado para prevenir coágulos sanguíneos;
- si alguno de sus familiares cercanos ha tenido alguna vez un coágulo sanguíneo en una pierna, pulmón u otro órgano;
- si tiene lupus eritematoso sistémico (LES);
- si tiene cáncer.
Para signos de un coágulo sanguíneo, ver “Interrumpa el uso de Estradot y acuda a un médico inmediatamente”.
Comparación
En mujeres en la cincuentena que no están tomando THS, se espera que una media de 4 a 7 de cada 1000 tengan un coágulo de sangre en una vena en un periodo de 5 años.
En mujeres en la cincuentena que toman THS combinada con estrógeno-progestágeno durante 5 años, habrá de 9 a 12 casos por cada 1000 usuarias (esto es, 5 casos adicionales).
En mujeres en la cincuentena a las que se les ha extraído el útero y que hayan sido tratadas con THS con estrógenos solos durante 5 años, habrá de 5 a 8 casos por cada 1000 usuarias (esto es, un caso adicional).
Enfermedad cardiaca (ataque al corazón)
No existe evidencia de que la THS prevenga un ataque al corazón.
Mujeres mayores de 60 años usuarias de THS combinada estrógeno-progestágeno tienen una probabilidad de desarrollar una enfermedad del corazón ligeramente superior a aquéllas no usuarias de THS.
En el caso de mujeres a las que se les haya extirpado el útero y que estén siendo tratadas con THS de estrógenos solos, no existe incremento del riesgo de desarrollar una enfermedad del corazón.
Accidente cerebrovascular
El riesgo de sufrir un accidente cerebrovascular es alrededor de 1.5 veces mayor para mujeres tratadas con THS que para no tratadas. El número de casos adicionales de accidente cerebrovascular debido al uso de THS incrementará con la edad.
Comparación
En mujeres en la cincuentena que no toman THS, un promedio de 8 por cada 1000 probablemente sufrirán un accidente cerebrovascular durante un periodo medio de 5 años. En mujeres en la cincuentena que estén siendo tratadas con THS, 11 por cada 1000 probablemente sufrirán un accidente cerebrovascular, durante un periodo de 5 años (esto es, 3 casos adicionales).
Otras condiciones
- La THS no previene la pérdida de memoria. Existe alguna evidencia de un mayor riesgo de pérdida de memoria en mujeres que empiezan la THS después de los 65 años. Hable con su médico para que le aconseje.
Otros medicamentos y Estradot
Algunos medicamentos pueden interferir con el efecto de Estradot. Esto puede provocar hemorragias irregulares. Esto ocurre con los siguientes medicamentos:
- Medicamentos para la epilepsia (como fenobarbital, fenitoína y, carbamazepina);
- Medicamentos para la tuberculosis (como rifampicina, rifabutina);
- Medicamentos para la infección por VIH (como nevirapina, efavirenz, ritonavir, nelfinavir);
- Preparados a base de plantas medicinales que contengan hierba de San Juan (Hypericum perforatum);
La THS puede afectar al funcionamiento de otros medicamentos:
- Un medicamento para la epilepsia (lamotrigina), ya que podría aumentar la frecuencia de los ataques.
- Otros medicamentos antiinfecciosos (como ketoconazol, eritromicina).
- Las pautas combinadas para el virus de la hepatitis C (VHC) ombitasvir/paritaprevir/ritonavir y dasabuvir con y sin ribavirina; glecaprevir/pibrentasvir o sofosbuvir/velpatasvir/voxilaprevir (ver sección 4.4), pueden provocar elevaciones en los resultados sanguíneos de la función hepática (aumento de la enzima hepática ALT) en mujeres que utilizan AHC que contiene etinilestradiol. Estradot contiene estradiol en lugar de etinilestradiol. Se desconoce si se puede producir un aumento de la enzima hepática ALT cuando se utiliza Estradot con esta pauta combinada para el VHC. Su médico le informará al respecto.
Por favor informe a su médico o farmacéutico si está tomando o ha tomado recientemente otros medicamentos, incluyendo los adquiridos sin receta, plantas medicinales u otros productos naturales. Su médico le informará al respecto.
Pruebas de laboratorio
Si necesita un análisis de sangre, comente a su médico o al personal de laboratorio que está usando Estradot, porque este medicamento puede afectar a los resultados de algunos análisis.
Embarazo y lactancia
Estradot es un medicamento únicamente para mujeres postmenopáusicas. Si se queda embarazada, interrumpa el tratamiento con Estradot y contacte con su médico.
No utilice Estradot si está embarazada o mientras esté en periodo de lactancia.
Conducción y uso de máquinas
Estradot no tiene efectos conocidos sobre la capacidad de conducir o manejar máquinas.
3. Cómo usar Estradot
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
Su médico intentará prescribirle la dosis más baja para tratar su síntoma durante el periodo de tiempo más corto posible. Hable con su médico si considera que esta dosis es demasiado fuerte o insuficiente.
Durante cuánto tiempo debe usar Estradot
Es importante que use la menor dosis eficaz posible y sólo mientras sea necesario.
Periódicamente, deberá comentar con su médico los posibles riesgos y beneficios asociados con el uso Estradot y si todavía necesita este tratamiento.
Cuándo empezar el tratamiento
- Si actualmente no está utilizando ningún tratamiento hormonal sustitutivo (parches o comprimidos), o si ha estado utilizando un producto de tratamiento hormonal sustitutivo continuo combinado (en el cual se administran estrógeno y progestágeno cada día sin interrupción), puede empezar a utilizar Estradot en cualquier día.
- Si va a cambiar desde un tratamiento hormonal sustitutivo cíclico o secuencial (en el cual el progestágeno se añade durante 12-14 días del ciclo), debe empezar a utilizar Estradot el día siguiente de completar su régimen anterior.
Cuándo aplicar Estradot
- Cada parche de Estradot debe cambiarse dos veces por semana (cada 3 a 4 días). Lo mejor es cambiarlo siempre los dos mismos días de la semana (por ejemplo lunes y jueves). El envase de Estradot contiene un calendario-control en la parte posterior que le ayudará a recordar su pauta. Marque la pauta de dos días por semana que desee seguir. Cambie siempre el parche los dos días de la semana que ha marcado.
- Deberá llevar el parche de Estradot de forma continuada hasta el momento de cambiarlo por otro parche nuevo.
Cualquier adhesivo que permanezca en la piel puede ser fácilmente eliminado mediante fricción. Si esto ocurriera, coloque un nuevo parche de Estradot en otra zona de la piel.
Mujeres a las que se les ha extirpado el útero
El parche de Estradot debe ser aplicado de forma continua sin interrupción. No es necesaria la adición de otro tipo de hormona llamada progestágeno, a menos que exista crecimiento del endometrio fuera del útero (endometriosis). Compruebe los riesgos a tener en cuenta con el tratamiento hormonal de sustitución en la sección 2, Advertencias y precauciones.
Mujeres que conservan el útero
Su médico le recetará otra hormona para tomar con Estradot, llamada progesterona, para reducir el riesgo de cáncer de útero. Si se aplica Estradot de forma continua sin interrupción, el comprimido de progesterona deberá tomarse durante al menos 12 – 14 días cada mes/ciclo de 28 días. Compruebe los riesgos a tener en cuenta con el tratamiento hormonal de sustitución en la sección 2, Advertencias y precauciones.
Puede sufrir sangrados irregulares o manchados durante los primeros meses de tratamiento. Si tiene sangrados abundantes o el sangrado o manchado continúa tras unos meses de tratamiento, hable con su médico para que pueda reevaluar su tratamiento si fuese necesario (ver sección 2, Hemorragias no esperadas).
Dónde aplicar Estradot
Aplicar el parche en la parte baja del abdomen, por debajo de la cintura. Evitar la cintura, ya que la ropa puede provocar que se despegue el parche. No aplique el parche en los pechos o cerca de los pechos.
Cuando cambie el parche, según su pauta de dos veces por semana, aplique el nuevo parche en una zona diferente. No aplique un nuevo parche en la misma zona durante el menos una semana.
Antes de aplicar Estradot, asegúrese que su piel está:
- limpia, seca y fresca,
- libre de cualquier polvo, aceite, crema, o loción,
- libre de cortes y/o irritaciones.
Cómo aplicar Estradot
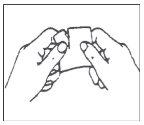
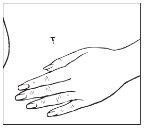
Cada parche se encuentra sellado individualmente en un sobre protector. Abrir el sobre por la ranura y sacar el parche (no utilice tijeras para abrir el sobre puesto que pueden dañar el parche).
| |
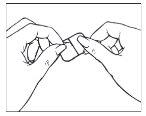
Una lámina protectora cubre la cara adherente del parche. Esta lámina debe ser despegada antes de aplicar el parche en la piel. Aplicar el parche inmediatamente tras abrir el sobre y sacar la lámina protectora. Coja el parche con la lámina protectora mirando hacia Vd. Despegue la mitad de la capa protectora y descártela. Intente evitar tocar la cara adherente del parche con los dedos.
| |
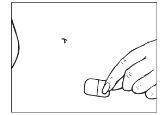
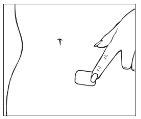
Coja la otra mitad de la lámina protectora, aplique la parte adhesiva del parche en una zona seca de la parte baja del abdomen. Presione la parte adhesiva contra la piel con el fin de que se adhiera correctamente, especialmente en los bordes. Elimine la otra parte de la lámina protectora.
| |
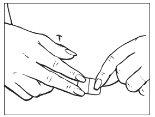
Sujete el borde recto de la lámina protectora y despéguelo del parche.
| |
Presione la parte adhesiva contra la piel con el fin de que se adhiera correctamente. Presione el parche firmemente en la piel con la palma de la mano durante al menos 10 segundos.
| |
Asegúrese que el parche haya sido colocado correctamente en la piel y pase los dedos por los bordes para comprobar un buen contacto entre el parche y la piel.
| |
Al cambiar el parche, despéguelo, y pliéguelo por la mitad con el lado pegajoso por dentro, Ver sección 5, “Conservación de Estradot” para obtener instrucciones para la eliminación del parche de manera segura. No tire los parches utilizados en el inodoro.
Información práctica adicional
Si el parche ha sido colocado correctamente no debería afectarle el baño, la natación, la ducha o el ejercicio. Si un parche se despega, por ejemplo durante el baño o la ducha, muévalo para eliminar el agua. Después de secar y dejar que se enfríe la piel, puede aplicar el mismo parche en una zona diferente de la piel de la parte baja del abdomen (ver “Dónde aplicar Estradot”).
Si el parche no se adhiere completamente a la piel, utilice un nuevo parche. No importa en qué día ocurra, vuelva a cambiar este parche el mismo día según la pauta inicial.
Al tomar el sol o utilizar un solarium, deberá cubrir el parche. Al bañarse, el parche lo puede llevar debajo del traje de baño.
Si necesita cirugía
Si va a someterse a una intervención quirúrgica, comente a su cirujano que está utilizando Estradot. Puede que necesite interrumpir el uso de Estradot entre 4 y 6 semanas antes de la operación para reducir el riesgo de formación de un coágulo sanguíneo (ver sección 2, Coágulos de sangre en una vena). Pregunte a su médico cuándo puede iniciar el tratamiento con Estradot de nuevo.
Si usa másEstradotdel que debe
Si ha tomado demasiado Estradot deberá quitarse el parche. Los síntomas de sobredosificación son normalmente dolor en los pechos y/o hemorragia vaginal. Es poco probable una sobredosis aguda debido a la vía de administración de Estradot (el parche libera el fármaco gradualmente). Si los síntomas persisten, deberá contactar con su médico.
Si olvidó usarEstradot
Si olvidó cambiar el parche, aplique otro parche tan pronto como se acuerde. No importa el día que esto ocurra, vuelva a cambiar el parche los mismos días que en la pauta inicial.
No tome una dosis doble para compensar la aplicación olvidada.
Si interrumpe el tratamiento conEstradot
La interrupción del tratamiento con Estradot puede aumentar el riesgo de sangrados irregulares o manchados. Informe a su médico si esto ocurre. Después de un periodo prolongado sin tratamiento, deberá consultar con su médico antes de empezar a utilizar el parche otra vez.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Estradot puede producir efectos adversos, aunque no todas las personas los sufran.
Las siguientes enfermedades se observan con más frecuencia en mujeres tratadas con THS en comparación con mujeres no tratadas con THS:
- cáncer de mama;
- crecimiento anormal de la pared interna del útero (hiperplasia de endometrio) o cáncer de la pared interna del útero (cáncer de endometrio) de endometrio;
- cáncer de ovario;
- coágulos de sangre en venas de las piernas o de los pulmones (tromboembolismo venoso);
- enfermedad del corazón;
- accidente cerebrovascular;
- pérdida de memoria probable si se empieza la THS a partir de los 65 años.
Para más información sobre estos efectos adversos, ver sección 2.
Algunos efectos adversos pueden ser graves
Los siguientes síntomas necesitan atención médica inmediata:
- Dolor en el pecho repentino;
- Dolor en el pecho que se esparce hacia el brazo o hacia el cuello;
- Dificultad para respirar;
- Hinchazón dolorosa y enrojecimiento de las piernas;
- Coloración amarilla de ojos y cara, oscurecimiento de la orina, picor en la piel (ictericia);
- Hemorragias o manchados no esperados después de utilizar Estradot durante algún tiempo o si esto continúa después de interrumpir el tratamiento;
- Cambio en los pechos, como alteración de la piel del pecho, cambios en los pezones, bultos que puede ver o notar (cáncer de mama);
- Periodos menstruales fuertes;
- Dolores de cabeza de tipo migrañosos no explicados.
Interrumpa el tratamiento con Estradotycontacteinmediatamentecon su médicosi sufre alguno de los efectos adversos mencionados anteriormente.
Compruebe los riesgos a tener en cuenta con el tratamiento hormonal de sustitución en la sección 2, Advertencias y precauciones
Otros efectos adversos
Estradot también puede producir los siguientes efectos adversos. Si considera que alguno de los efectos adversos que sufre es grave, informe asu médico o farmacéutico.
Efectos adversos muy frecuentes,pueden afectar a más de 1 de cada 10 personas:
Dolor de cabeza, reacciones en la piel en el lugar de aplicación del parche (que incluyen irritación, ardor, erupción, sequedad, sangrado, morados, inflamación, hinchazón, pigmentación de la piel, urticaria, y ampollas), tensión y dolor en los pechos, dolor menstrual, alteración menstrual.
Frecuentes,pueden afectar a hasta 1 de cada 10 personas:
Depresión, nerviosismo, cambios de humor, insomnio, nausea, mala digestión, diarrea, dolor abdominal, sensación de hinchazón, acné, erupción, piel seca, picor, crecimiento de los pechos, periodos menstruales fuertes, descarga de un líquido viscoso blanco o amarillento de la vagina, sangrados irregulares, contracciones uterinas graves, inflamación de la vagina, crecimiento anormal del útero (hiperplasia de endometrio), dolor (p.ej. dolor de espalda, brazos, piernas, muñecas, tobillos), debilidad, retención de líquidos (edema) en las extremidades (brazos y piernas), cambios de peso.
Poco frecuentes,pueden afectar a hasta 1 de cada 100 personas:
Migraña, mareo, aumento de la tensión arterial, vómitos, coloración de la piel, alteración de la función del hígado.
Raros,pueden afectar a hasta 1 de cada 1000 personas:
Hormigueo o adormecimiento de manos y pies, coágulos en la sangre, cálculos en la vesícula biliar, pérdida de cabello, debilidad muscular, crecimiento benigno del útero, quistes próximos a los tubos uterinos, pólipos (pequeños bultos) en el cérvix del útero (cuello uterino), cambios en el deseo sexual, reacciones alérgicas como erupciones.
Muy raros,pueden afectar a hasta 1 de cada 10000 personas:
Urticaria, signos de reacción alérgica graves (que incluyen dificultades para respirar; hinchazón de la cara, la lengua, la garganta o de la piel; mareo y urticaria), disminución de la tolerancia a los hidratos de carbono, movimientos involuntarios que pueden afectar los ojos, la cabeza y el cuello, malestar con el uso de lentes de contacto, reacciones de la piel graves, crecimiento excesivo de pelo.
Efectos adversos de frecuencia no conocida(no puede ser estimada con los datos disponibles):
Cáncer de mama, prueba de función hepática anormal, inflamación alérgica de la piel, bultos en el pecho (no cancerígenos).
Los siguientes efectos adversos se han comunicado asociados con otros tratamientos hormonales sustitutivos:
- enfermedad de la vesícula biliar
- diversas alteraciones de la piel:
- decoloración de la piel, especialmente de la cara o cuello, conocida como “manchas del embarazo” (cloasma);
- nódulos dolorosos y enrojecidos en la piel (eritema nodoso);
- erupción cutánea con enrojecimiento en forma de diana o llagas (eritema multiforme);
- disminución de la memoria o la capacidad mental (posible demencia)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano (https://www.notificaram.es). Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Estradot
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- Conservar Estradot en el sobre original, en lugar fresco y seco. Una vez abierto o una vez se haya retirado la lámina protectora, aplicar el parche sobre la piel inmediatamente.
- No refrigerar ni congelar Estradot.
- No utilice este medicamento después de la fecha de caducidad que aparece en el sobre después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
- No utilice este medicamento si observa que el envase está dañado o muestra signos de manipulación.
- Después de quitarse el parche, pliéguelo por la mitad con la parte adhesiva para dentro y manténgalo en un lugar seguro fuera del alcance de los niños. Los parches transdérmicos usados o sin usar deben eliminarse de acuerdo a la normativa local o devueltos a la farmacia, preferiblemente en su envase original.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Estradot
Cada parche de 75 microgramos/24 horas parche transdérmico contiene 1,17 mg de estradiol (como hemihidrato) y libera unos 75 microgramos de estradiol cada 24 horas.
- El principio activo es estradiol (como hemihidrato).
- Los demás componentes de la lámina adhesiva del parche son: adhesivo acrílico, silicona adhesiva, alcohol oleico, dipropilenglicol, povidona (E1201).
- La capa de soporte es un copolímero de etileno/vinilacetato y un copolímero laminado de cloruro de vinilideno/acrilato de metilo.
- La lámina protectora (que se desprende antes de aplicar el parche) es una película de poliéster recubierta de fluoropolímero.
Aspecto del producto y contenido del envase
Estradot 75 es un parche rectangular de 7,5 cm2 con bordes redondeados, compuesta por una capa adhesiva sensible a la presión que contiene estradiol, capa de soporte traslúcida en una cara y una lámina protectora en la otra.
Estradot se encuentra disponible en cuatro concentraciones diferentes: 25, 37,5 50 y 75 microgramos/24 horas. Puede que no todas las concentraciones estén disponibles.
Estradot está disponible en envases de 2, 8, 24 y 26 parches. Puede que no todos los formatos estén disponibles.
Titular de la Autorización de Comercialización
BEXAL FARMACÉUTICA, S.A.
Centro Empresarial Parque Norte
Edificio Roble
C/ Serrano Galvache, 56
28033 Madrid
España
Responsable de la fabricación
Novartis Farmacéutica, S.A.
Gran Vía de les Corts Catalanes, 764
08013 Barcelona
España
Novartis Pharma GmbH
Roonstrasse 25
D-90429 Nürnberg
Alemania
Este medicamento está autorizado en los Estados Miembros del Espacio Económico Europeo y en el Reino Unido (Irlanda del Norte) con los siguientes nombres:
Austria: Estradot
Dinamarca: Vivelle dot
Finlandia: Estradot
Francia: Vivelledot
Croacia: Estradot
Islandia: Vivelle dot
Irlanda: Estradot
Noruega: Estradot
Portugal: Estradot
España: Estradot
Suecia: Estradot
Reino Unido (Irlanda del Norte): Estradot
Fecha de la última revisión de este prospecto:mayo 2025
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ESTRADOT 75 microgramos/24 HORAS, PARCHE TRANSDERMICOForma farmacéutica: GEL, 0,5 mgPrincipio activo: EstradiolFabricante: Orion CorporationRequiere recetaForma farmacéutica: GEL, 1 mgPrincipio activo: EstradiolFabricante: Orion CorporationRequiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 3 mgPrincipio activo: EstradiolFabricante: Merus Labs Luxco Ii S.À.R.L.Requiere receta
Médicos online para ESTRADOT 75 microgramos/24 HORAS, PARCHE TRANSDERMICO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ESTRADOT 75 microgramos/24 HORAS, PARCHE TRANSDERMICO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes