GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL

Pergunte a um médico sobre a prescrição de GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL

Como usar GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL
Introdução
Prospecto: informação para o utilizador
GONAPEPTYL DEPOT 3,75 miligramas
Pó e dissolvente para suspensão injetável
Triptorelina
Leia todo o prospecto atentamente antes de começar a usar o medicamento
- Conserva este prospecto, pois pode ter que voltar a lê-lo. Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Se considera que algum dos efeitos adversos que sofre é grave ou se apercebe de qualquer efeito adverso não mencionado neste prospecto, informe o seu médico ou farmacêutico.
Conteúdo do prospecto
- O que é GONAPEPTYL DEPOT e para que é utilizado
- Antes de começar a usar GONAPEPTYL DEPOT
- Como usar GONAPEPTYL DEPOT
- Posíveis efeitos adversos
- Conservação de GONAPEPTYL DEPOT
- Informação adicional
1. O que é GONAPEPTYL DEPOT e para que é utilizado
GONAPEPTYL Depot contém triptorelina (como acetato de triptorelina). A triptorelina pertence ao grupo de medicamentos chamados análogos da GnRH. Uma das suas ações é diminuir a produção de hormonas sexuais no corpo.
É utilizado:
No homem:
- Tratamento de cancro da próstata localmente avançado ou metastático dependente das hormonas.
Na mulher:
Para suprimir os níveis de hormonas ováricas para:
- Reduzir o tamanho dos miomas uterinos, (comumente conhecidos como fibromas) que são tumores não cancerígenos surgidos do miométrio (camada lisa do músculo) do útero.
- Tratar a endometriose (presença de tecido uterino fora do útero).
Nas crianças:
- Tratamento da puberdade precoce central (puberdade que ocorre prematuramente mas com os cambios físicos e hormonais de puberdade normais).
2. Antes de usar GONAPEPTYL DEPOT
Não deve usar GONAPEPTYL Depot:
- Se é alérgico à triptorelina ou a qualquer um dos outros componentes de GONAPEPTYL Depot.
- Se é alérgico à hormona liberadora de gonadotropinas (GnRH) ou a qualquer outro análogo de GnRH.
Nas mulheres:
- Se está grávida ou está a amamentar o seu filho.
Tenha especial cuidado com GONAPEPTYL Depot
No homem e na mulher:
- Foram comunicados casos de depressão em pacientes tratados com Gonapeptyl que podem chegar a ser graves. Se está a tomar Gonpapetyl e desenvolve humor depressivo, informe o seu médico.
- GONAPEPTYL Depot pode dar origem a alterações de humor.
- O tratamento com GONAPEPTYL Depot em raros casos pode dar origem a hemorragias cerebrais (apoplexia hipofisária). Informe o seu médico imediatamente se tiver dor de cabeça repentina, vómitos ou incómodos na visão.
- O tratamento com GONAPEPTYL Depot pode dar origem a perda de massa óssea dos ossos que aumenta o risco de lesão dos ossos.
- Se tiver um risco adicional de perda de massa óssea dos ossos (osteoporose) deve informar o seu médico antes de usar GONAPEPTYL Depot. Os factores de risco incluem:
- Se qualquer membro da sua família próxima tem perda de massa óssea dos ossos.
- Se bebe quantidades excessivas de álcool, tem uma dieta pobre e/ou fuma muito.
- Se além disso está a ser tratado com certos medicamentos que possam afectar a resistência do osso.
No homem:
Informe o seu médico:
- se tem dor nos seus ossos, ou dificuldade para urinar.
- se tem tumor espinal secundário ou tumor do tracto urinário.
- se está castrado.
- se lhe foi diagnosticada diabetes.
- se tem um risco alto de doenças do coração, como pressão arterial alta diagnosticada ou problemas com o ritmo do coração (arritmia).
- se tem algum problema cardíaco ou vascular, incluindo problemas do ritmo cardíaco (arritmia), ou se está a ser tratado com medicamentos para estas condições. Pode aumentar o risco de problemas do ritmo cardíaco quando se utiliza Gonapeptyl.
Durante o tratamento:
Durante o início do tratamento com GONAPEPTYL Depot pode experimentar um agravamento dos sintomas da sua doença.
Consulte o seu médico se qualquer um dos sintomas da sua doença se agravarem.
Nas mulheres:
Informe o seu médico
- se experimenta sangramento a meio do ciclo durante o tratamento (excepto durante o primeiro mês).
Durante o tratamento:
Devem ser utilizadas medidas contraceptivas não hormonais, como o preservativo ou diafragma, durante o primeiro mês após a primeira injeção. Devem também ser utilizadas a partir da semana 4 após a última injeção até que se restabeleça o seu período (menstruação).
A sua menstruação será interrompida durante o tratamento. Uma vez finalizado o tratamento, os seus períodos (menstruação) se restabelecerão 7-12 semanas após a injeção final.
Se os seus períodos (menstruação) persistirem durante o tratamento, por favor informe o seu médico.
Nas crianças:
- O tratamento só deve ser iniciado em meninas com idade inferior a 9 anos e em meninos com idade inferior a 10 anos.
Informe o seu médico:
- Se o seu filho sofre dor de cabeça intensa ou recorrente, problemas de visão e zumbidos ou assobios nos ouvidos, entre em contacto com um médico imediatamente (ver seção 4).
Durante o tratamento:
Durante o primeiro mês de tratamento, as meninas podem experimentar episódios de sangramento vaginal de leve a moderado.
Uma vez finalizado o tratamento, ocorrerá o desenvolvimento das características da puberdade. Na maioria das meninas, a menstruação ocorrerá transcorrido um ano desde a finalização do tratamento, e na maioria dos casos será regular.
Para qualquer possível efeito adverso por favor ver a seção 4.
Uso de outros medicamentos
Informe o seu médico ou farmacêutico se está a tomar ou tomou recentemente qualquer outro medicamento, mesmo os adquiridos sem receita.
GONAPEPTYL Depot pode interferir com alguns medicamentos utilizados para tratar problemas do ritmo cardíaco (por exemplo, quinidina, procainamida, amiodarona e sotalol) ou pode aumentar o risco de problemas do ritmo cardíaco quando se utiliza com outros medicamentos (por exemplo, metadona (utilizada para o alívio da dor e para a desintoxicação de adicção a drogas), moxifloxacino (um antibiótico), os antipsicóticos utilizados para doenças mentais graves.
Condução e uso de máquinas
Não se conhecem efeitos sobre a capacidade de conduzir e utilizar máquinas.
No entanto, não pode excluir-se que a capacidade de conduzir ou usar máquinas possa estar afectada durante o tratamento devido a algum dos efeitos adversos (tonturas, perturbações do sono/insónia e alteração da visão do olho). Tome precaução extra se experimenta estes efeitos adversos.Condução e uso de máquinas
Gravidez e amamentaçãoNão se conhecem efeitos sobre a capacidade de conduzir e utilizar máquinas.
No entanto, não pode excluir-se que a capacidade de conduzir ou usar máquinas possa estar afectada durante o tratamento devido a algum dos efeitos adversos (tonturas, perturbações do sono/insónia e alteração da visão do olho). Tome precaução extra se experimenta estes efeitos adversos.
Gonapapetyl não deve ser utilizado durante a gravidez e a amamentação (ver a seção “Não deve usar GONAPEPTYL Depot”). Se acredita estar grávida, o seu médico deve descartar a gravidez antes de usar Gonapeptyl Depot.
As mulheres férteis devem utilizar métodos contraceptivos não hormonais eficazes, tais como o preservativo ou diafragma, durante o tratamento com Gonapeptyl Depot até que a menstruação se reanude.
Condução e uso de máquinas
Não se conhecem efeitos sobre a capacidade de conduzir e utilizar máquinas.
No entanto, não pode excluir-se que a capacidade de conduzir ou usar máquinas possa estar afectada durante o tratamento devido a algum dos efeitos adversos (tonturas, perturbações do sono/insónia e alteração da visão do olho). Tome precaução extra se experimenta estes efeitos adversos.
Gonapapetyl não deve ser utilizado durante a gravidez e a amamentação (ver a seção “Não deve usar GONAPEPTYL Depot”). Se acredita estar grávida, o seu médico deve descartar a gravidez antes de usar Gonapeptyl Depot.
As mulheres férteis devem utilizar métodos contraceptivos não hormonais eficazes, tais como o preservativo ou diafragma, durante o tratamento com Gonapeptyl Depot até que a menstruação se reanude.
Condução e uso de máquinas
Não se conhecem efeitos sobre a capacidade de conduzir e utilizar máquinas.
No entanto, não pode excluir-se que a capacidade de conduzir ou usar máquinas possa estar afectada durante o tratamento devido a algum dos efeitos adversos (tonturas, perturbações do sono/insónia e alteração da visão do olho). Tome precaução extra se experimenta estes efeitos adversos.
Gonapeptyl não deve ser utilizado durante a gravidez e a amamentação (ver seção “Não deve usar GONAPEPTYL Depot”). Se acredita estar grávida, o seu médico deve descartar a gravidez antes de usar Gonapeptyl Depot.
As mulheres férteis devem utilizar métodos contraceptivos não hormonais eficazes, tais como o preservativo ou diafragma, durante o tratamento com Gonapeptyl Depot até que a menstruação se reanude.
Gonapapetyl não deve ser utilizado durante a gravidez e a amamentação (ver a seção “Não deve usar GONAPEPTYL Depot”). Se acredita estar grávida, o seu médico deve descartar a gravidez antes de usar Gonapeptyl Depot.
As mulheres férteis devem utilizar métodos contraceptivos não hormonais eficazes, tais como o preservativo ou diafragma, durante o tratamento com Gonapeptyl Depot até que a menstruação se reanude.
Condução e uso de máquinas
Não se conhecem efeitos sobre a capacidade de conduzir e utilizar máquinas.
No entanto, não pode excluir-se que a capacidade de conduzir ou usar máquinas possa estar afectada durante o tratamento devido a algum dos efeitos adversos (tonturas, perturbações do sono/insónia e alteração da visão do olho). Tome precaução extra se experimenta estes efeitos adversos.
3. Como usar GONAPEPTYL DEPOT
O pó e o dissolvente são misturados e injetados normalmente pelos profissionais de saúde.
Dependendo da indicação do seu tratamento, será administrada a dose adequada mediante injeção intramuscular (no músculo) ou injeção subcutânea (só debaixo da pele).
No homem:
- É administrada normalmente uma injeção de GONAPEPTYL Depot cada 4 semanas como tratamento a longo prazo.
Nas mulheres:
- É administrada normalmente uma injeção de GONAPEPTYL Depot cada 4 semanas durante um máximo de 6 meses.
- O tratamento deve ser iniciado durante os primeiros cinco dias do ciclo menstrual.
Nas crianças:
- No início do tratamento, deve ser administrada uma injeção de triptorelina nos dias 0, 14 e 28.
- A dose é ajustada de acordo com o peso da criança. Crianças com peso inferior a 20 quilogramas são administradas 1,875 miligramas (1/2 dose), crianças entre 20-30 quilogramas são administradas 2,5 miligramas (2/3 dose) e crianças com peso superior a 30 quilogramas são administradas 3,75 miligramas.
- Posteriormente, são administradas as injeções cada 3-4 semanas, em função do seu efeito.
A duração do tratamento deve ser supervisionada pelo seu médico.
Se usar mais GONAPEPTYL Depot do que o que devia:
Não é muito provável que seja administrada mais GONAPEPTYL Depot do que o que você deveria ter recebido. Se foi administrada mais GONAPEPTYL Depot do que o que devia, informe o seu médico ou farmacêutico imediatamente.
Se interromper o tratamento com GONAPEPTYL Depot
O tratamento com GONAPEPTYL Depot deve ser apenas suspenso sob conselho do seu médico. Se tiver alguma outra dúvida sobre o uso deste produto, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Tal como todos os medicamentos, GONAPEPTYL Depot pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Geral (todos os pacientes):
Se experimentar inchaço do rosto, lábios, boca ou garganta que possa causar dificuldade em engolir ou respirar, consulte o seu médico ou vá ao serviço de saúde mais próximo.
Foram relatados casos de aumento de tumores hipofisários existentes durante o tratamento com agonistas LH-RH, no entanto, estes não foram observados com o tratamento de triptorelina.
Em homens:
Devido ao aumento dos níveis de testosterona no início do tratamento, inicialmente podem piorar os sintomas para os quais está sendo tratado (ou seja, obstrução urinária, dor vertebral, compressão da medula espinhal, fraqueza muscular e edemas nas pernas e fraqueza e formigamento nos pés e nas mãos).
Muito frequentes, mais de 1 paciente de cada 10 pacientes tratados:a maioria dos efeitos adversos de GONAPEPTYL Depot em homens ocorre devido à descida dos níveis de testosterona. Pode ocorrer impotência, diminuição da libido, sofocos, dor óssea, dificuldade e dor ao urinar.
Frequentes, entre 1 e 10 pacientes de cada 100 pacientes tratados:reação alérgica, humor depressivo, mudanças de humor, depressão, distúrbios do sono, náuseas, dor muscular e articular, cansaço, reação no local de injeção, dor no local de injeção, irritabilidade, sudorese excessiva, dor de cabeça e aumento do volume do peito em homens.
Pouco frequentes, entre 1 e 10 pacientes de cada 1000 pacientes tratados:elevação de algumas enzimas hepáticas, reação anafiláctica, atrofia testicular, pressão sanguínea alta, diminuição do apetite, secura da boca, dor abdominal superior, piora do asma, mudanças de peso, embolia, perda de cabelo e menor crescimento do cabelo.
Frequência desconhecida, não pode ser estimada a partir dos dados disponíveis:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Em mulheres:
Muito frequentes, mais de 1 paciente de cada 10 pacientes tratados:diminuição da libido, mudanças de humor, distúrbios do sono, sofocos, dor abdominal, dor óssea, sudorese excessiva, sangramento/vazamento vaginal, secura vulvovaginal, dor durante o ato sexual, menstruação dolorosa, aumento do tamanho dos ovários, dor pélvica, fraqueza e dor de cabeça.
Frequentes, entre 1 e 10 pacientes de cada 100 pacientes tratados:reação alérgica, humor depressivo, depressão, náuseas, dor muscular e articular, cansaço, reação no local de injeção, dor no local de injeção, irritabilidade.
Pouco frequentes, entre 1 e 10 pacientes de cada 1000 pacientes tratados:reação anafiláctica, distúrbios visuais, sensação de formigamento, hormigueio ou entorpecimento, dor nas costas, aumento do colesterol no sangue, elevação de algumas enzimas hepáticas.
Frequência desconhecida, não pode ser estimada a partir dos dados disponíveis:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Em crianças:
Frequentes, entre 1 e 10 pacientes de cada 100 pacientes tratados:mudanças de humor, depressão.
Pouco frequentes, entre 1 e 10 pacientes de cada 1000 pacientes tratados:em meninas pode ocorrer sangramento ou fluxo vaginal. Foram vistos náuseas, vômitos e reação anafiláctica.
Frequência desconhecida, não pode ser estimada a partir dos dados disponíveis:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de GONAPEPTYL DEPOT
Mantenha fora do alcance e da vista das crianças.
Não utilize GONAPEPTYL Depot após a data de validade que aparece no envase. A data de validade é o último dia do mês que se indica.
Conservar na geladeira (entre 2 ?C e 8 ?C). Conservar no envase original.
Os medicamentos não devem ser jogados nos deságues nem na lixeira. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, você ajudará a proteger o meio ambiente.
6. Informação adicional
Composição de GONAPEPTYL DEPOT
- Em cada seringa pré-carregada, o pó contém 4,12 miligramas de acetato de triptorelina que equivale a 3,75 miligramas de princípio ativo, triptorelina.
- Os demais componentes são: Poli-(d,l láctido coglicólido), dicaprilocaprato de propilenglicol.
O solvente contém:
- Dextrano 70, polissorbato 80, cloreto de sódio, fosfato de hidrogênio sódico dihidrato, hidróxido de sódio e água para injeção.
Este produto contém menos de 1 milimol de sódio (3,69 miligramas/mililitro ou 0,160 milimoles/mililitro) por dose, ou seja, está essencialmente “livre de sódio”.
Aspecto de Gonapeptyl Depot e conteúdo do envase
Apresenta-se em envases de 1 conjunto de: 1 ou 3 pares de seringas pré-carregadas (pó e solvente).
Nem todas as apresentações estão disponíveis em Espanha.
Titular da autorização de comercialização e responsável pela fabricação:
Titular da autorização de comercialização:
FERRING S.A.U.
C/ do Arquiteto Sánchez Arcas 3, 1º
28040 Madrid.- Espanha
Responsável pela fabricação:
FERRING GmbH
Wittland 11,
D-24109 Kiel
Alemanha.
Este medicamento está autorizado nos estados membros do Espaço Econômico Europeu com os seguintes nomes:
GONAPEPTYL DEPOT (Bélgica, Grécia, Itália, Luxemburgo, Holanda, Suécia, Espanha, Portugal, Reino Unido), GONAPEPTYL 3.75 mg (França), GONAPEPTYL DEPOT 3.75 mg (Irlanda), DECAPEPTYL DEPOT (República Checa, Dinamarca, Islândia, Estônia, Alemanha, Letônia, Lituânia, Noruega, Polônia, Eslováquia), DECAPEPTYL DEPOT 3.75 (Finlândia), DECAPEPTYL N (Alemanha), UROPEPTYL DEPOT (Alemanha), DECAPEPTYL GYN (Alemanha), GYNOPEPTYL (Alemanha), DECAPEPTYL CR 3.75 (Holanda), DECAPEPTYL DEPOT-Retardmikrokapseln und Suspensionsmittel für Einmalspritzen (Áustria), DECAPEPTYL DEPOT injection (Hungria), DECAPEPTYL Retard injectionspräparat i.m/s.c (Suíça).
Este prospecto foi aprovado em Março de 2023.
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/
A seguinte informação está destinada apenas a profissionais de saúde:
INSTRUÇÕES DE USO
Informação importante:
- Guarde Gonapeptyl Depot no embalagem dentro da geladeira.
- Certifique-se de injetar Gonapeptyl Depot nos 3 minutos posteriores à reconstituição.
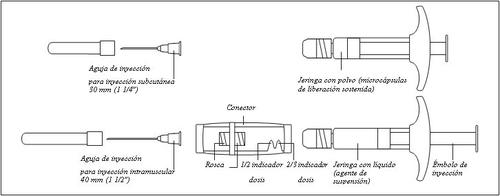
Descrição geral dos componentes de Gonapeptyl Depot:
- Preparação
Para garantir a correta preparação da suspensão, sigam estritamente as seguintes instruções:
A | B | C | |||
|
|
| |||
Certifique-se de não tocar os fios do conector | Certifique-se de não empurrar o êmbolo de injeção. | Sempre conecte a seringa com pó ao conector antes de conectar a seringa com líquido. |
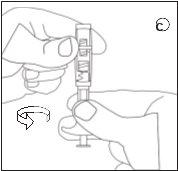
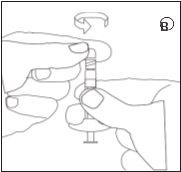
D | E | ||||
|
| CONTINUA NA PÁGINA POSTERIOR GIRAR FOLHA | |||
Certifique-se de não empurrar o êmbolo de injeção. |
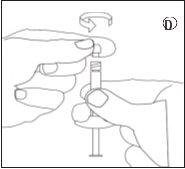
- Reconstituição
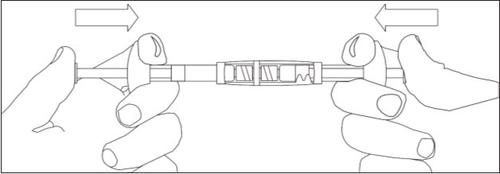
Para misturar a suspensão:
- Injetar todo o líquido na seringa com o pó.
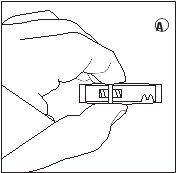
- Empurrar lentamente a suspensão para frente e para trás nas duas seringas até que a suspensão seja de cor branca leitosa homogênea a ligeiramente amarelada. Tenha cuidado para manter as seringas retas; não as incline.
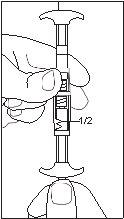
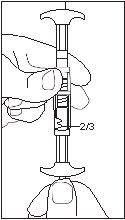
1/2 ou 2/3 dose para crianças:Use os indicadores de dose no conector para medir 1/2 ou 2/3 de dose: • Certifique-se de que a suspensão está na seringa conectada ao lado do conector sem indicadores de dose. • Girar as seringas para uma posição vertical com a seringa que contém a suspensão na parte superior. • Esperar alguns segundos para deixar que a espuma se separe. • Puxar lentamente o êmbolo de injeção da seringa vazia para baixo até que a suspensão alcance o indicador 1/2 ou 2/3. |
1/2 DOSE |
2/3 DOSE |
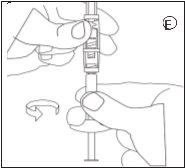
3.Injeção
- Desenroscar a seringa com a suspensão pronta para injeção do conector.
- Enroscar a agulha de injeção na seringa.
- Injetar a suspensão em 3 minutos.

Gonapeptyl Depot é apenas para um único uso e qualquer suspensão não utilizada deve ser descartada.
- País de registo
- Disponibilidade em farmáciasProblema de disponibilidade reportado
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVELForma farmacêutica: INJETÁVEL, 0,1 mgSubstância ativa: triptorelinFabricante: Ipsen Pharma S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, 3,75 mg de triptorelina/frascoSubstância ativa: triptorelinFabricante: Ipsen Pharma S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, 22.5 mgSubstância ativa: triptorelinFabricante: Ipsen Pharma S.A.U.Requer receita médica
Alternativas a GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL em Polónia
Alternativa a GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL em Ukraine
Médicos online para GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de GONAPEPTYL DEPOT 3,75 mg PÓ E SOLVENTE PARA SUSPENSÃO INJETÁVEL – sujeita a avaliação médica e regras locais.