

FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA

Pergunte a um médico sobre a prescrição de FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA

Como usar FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA
Introdução
Prospecto:informação para o paciente
Fulvestrant STADAFARMA 250 mg solução injetávelem seringa precarregada EFG
Leia todo o prospecto atentamente antes de receber este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver Seção 4.
Conteúdo do prospecto
- O que é Fulvestrant Stadafarma e para que é utilizado
- O que precisa saber antes de receber Fulvestrant Stadafarma
- Como é administrado Fulvestrant Stadafarma
- Possíveis efeitos adversos
5 Conservação de Fulvestrant Stadafarma
- Conteúdo do envase e informação adicional
1. O que é Fulvestrant Stadafarma e para que é utilizado
Fulvestrant Stadafarma contém o princípio ativo fulvestrante, que pertence ao grupo de bloqueantes de estrógeno. Os estrógenos, um tipo de hormonas sexuais femininas, podem estar em alguns casos implicados no desenvolvimento do cancro da mama.
Fulvestrante é utilizado:
- só, para tratar mulheres pós-menopáusicas com um tipo de cancro da mama chamado cancro da mama com receptor de estrógeno positivo, que está localmente avançado ou que se espalhou para outras partes do corpo (metastásico) ou,
- em combinação com palbociclib para tratar mulheres com um tipo de cancro da mama chamado cancro da mama com receptor hormonal positivo, cancro da mama com receptor 2 do factor de crescimento epidérmico humano negativo, que está localmente avançado ou que se espalhou para outras partes do corpo (metastásico). As mulheres que não tenham chegado à menopausa também serão tratadas com um medicamento chamado agonista da hormona liberadora de hormona luteinizante (LHRH).
Fulvestrante pode ser administrado em combinação com palbociclib. É importante que você leia também o prospecto de palbociclib. Se tiver alguma pergunta sobre palbociclib, consulte o seu médico.
2. O que precisa saber antes de receber Fulvestrant Stadafarma
Não deve receberFulvestrant Stadafarma:
- se é alérgico a fulvestrante ou a qualquer um dos outros componentes deste medicamento (incluídos na seção 6)
- se está grávida ou em período de amamentação
- se apresenta problemas hepáticos graves
Advertências e precauções
- Consulte o seu médico, farmacêutico ou enfermeiro antes de receber fulvestrante se tiver algum dos problemas seguintes:
- problemas de rim ou fígado
- contagem baixa de plaquetas (que ajudam à coagulação do sangue) ou alterações hemorrágicas
- problemas prévios de coágulos sanguíneos
- osteoporose (perda de densidade óssea)
- alcoolismo
Crianças e adolescentes
Fulvestrante não é indicado em crianças e adolescentes menores de 18 anos.
Outros medicamentos eFulvestrant Stadafarma
Informa ao seu médico ou farmacêutico se está tomando, tomou recentemente ou possa ter que tomar qualquer outro medicamento.
Em particular, deve dizer ao seu médico se está utilizando anticoagulantes (medicamentos para prevenir os coágulos sanguíneos).
Gravidez e amamentação
Não deve utilizar fulvestrante se está grávida. Se pode engravidar, deve utilizar um método anticonceptivo eficaz enquanto estiver em tratamento com fulvestrante e durante dois anos após a sua última dose.
Não deve amamentar enquanto estiver em tratamento com fulvestrante.
Condução e uso de máquinas
Não se espera que fulvestrante afete a sua capacidade para conduzir ou utilizar máquinas. No entanto, se se sentir cansada após o tratamento, não conduza nem utilize máquinas.
Uso em desportistas
Este medicamento contém fulvestrante que pode produzir um resultado positivo nos testes de controlo de dopagem.
Este medicamento contém 1000mg de álcool (etanol)em cada dose de 2 x 5 ml de solução injetável. A quantidade presente em 2 x 5 ml deste medicamento é equivalente a menos de 20 ml de cerveja ou 8 ml de vinho.
A pequena quantidade de álcool presente neste medicamento não tem nenhum efeito apreciável.
Este medicamento é prejudicial para pessoas que padecem de alcoolismo.
O conteúdo em álcool deve ser tido em conta no caso de grupos de alto risco, como pacientes com doenças do fígado, ou epilepsia.
Este medicamentocontém 500mg de álcool benzílicoem cada injeção de 5 ml, o que equivale a 100 mg/ml. O álcool benzílico pode provocar reações alérgicas. Consulte o seu médico ou farmacêutico se tiver algum problema de fígado ou rim. Deve fazê-lo porque, se receber quantidades abundantes de álcool benzílico, estas podem acumular-se no seu corpo e provocar efeitos adversos (o que se conhece como «acidose metabólica»).
Este medicamento contém 750mg de benzoato de benziloem cada injeção de 5 ml, o que equivale a 150 mg/ml.
3. Como usar Fulvestrant Stadafarma
A dose recomendada é 500 mg de fulvestrante (duas injeções de 250 mg/5 ml) administrados uma vez ao mês, com uma dose adicional de 500 mg às 2 semanas da dose inicial.
O seu médico ou enfermeiro administrará fulvestrante mediante uma injeção intramuscular lenta em cada um dos seus glúteos.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
Em caso de sobredose ou ingestão acidental, consulte imediatamente o seu médico ou farmacêutico ou ligue para o Serviço de Informação Toxicológica, telefone: 91 562 04 20, indicando o medicamento e a quantidade ingerida.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Informa imediatamente ao seu médico se experimenta algum dos efeitos adversos seguintes, porque pode precisar de tratamento médico imediato:
- Reações alérgicas (hipersensibilidade), incluindo inchaço do rosto, lábios, língua e/ou garganta, que podem ser sintomas de reações anafilácticas
- Tromboembolismo (aumento do risco de coágulos sanguíneos)*
- Inflamação do fígado (hepatite)
- Falha hepática
Informa imediatamente ao seu médico, farmacêutico ou enfermeiro se nota algum dos seguintes efeitos adversos:
- Muito frequentes(podem afetar mais de 1 de cada 10 pessoas)
- Reações no local da injeção, como dor e/ou inflamação
- Níveis anormais de enzimas hepáticas (em análise de sangue)*
- Náuseas (sensação de mal-estar)
- Debilidade, cansaço*
- Dor articular e musculoesquelética
- Sofocos
- Erupção cutânea
Todos os efeitos adversos restantes:
- Frequentes(podem afetar até 1 de cada 10 pessoas)
- Dor de cabeça
- Vómitos, diarreia ou perda de apetite*
- Infecções do trato urinário
- Dor de costas*
- Aumento de bilirrubina (um pigmento da bile produzido pelo fígado)
- Níveis diminuídos de plaquetas (trombocitopenia)
- Hemorragia vaginal
- Dor lombar que se reflete em um lado da perna (ciática)
- Debilidade súbita, entorpecimento, formigamento ou perda de movimento na sua perna, especialmente em um só lado do corpo, problemas súbitos para caminhar ou de equilíbrio (neuropatia periférica)
Pouco frequentes(podem afetar até 1 de cada 100 pessoas)
- Fluxo vaginal espesso, branco e candidíase (infecção)
- Hematoma e hemorragia no local da injeção
- Aumento de gama-GT, uma enzima hepática que se identifica em uma análise de sangue
- Entorpecimento, formigamento e dor
- Reações anafilácticas
- Inclui efeitos adversos para os quais não se pode avaliar o papel exato de fulvestrante devido à doença subjacente.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do sistema nacional de notificação incluído Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Fulvestrant Stadafarma
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no envase ou nas etiquetas das seringas após a abreviatura CAD. A data de validade é o último dia do mês que se indica.
Conservar e transportar em frigorífico (entre 2 ºC e 8 ºC).
As desvições de temperatura de entre 2 ºC e 8 ºC devem ser limitadas. Isso inclui evitar a conservação a temperaturas superiores a 25 ºC, e que não exceda um período de 28 dias no qual a temperatura média de conservação do medicamento esteja por debaixo de 25 ºC (mas por cima de entre 2 ºC e 8 ºC). Após produzir-se alguma desviação de temperatura, deve devolver imediatamente o medicamento às condições de conservação recomendadas (conservação e transporte refrigerado entre 2 ºC e 8 ºC). As desvições de temperatura têm um efeito acumulativo na qualidade do medicamento e não se deve superar o período de 28 dias durante o período de validade de 36 meses de fulvestrante injetável. A exposição a temperaturas inferiores a 2 ºC não danificará o medicamento desde que este não seja conservado por debaixo de -20 ºC.
Conservar a seringa precarregada no embalagem original para protegê-la da luz.
O seu profissional de saúde será o responsável pela conservação, uso e eliminação corretos de Fulvestrant Stadafarma 250 mg solução injetável em seringa precarregada EFG.
Este medicamento pode apresentar um risco para o meio aquático. Os medicamentos não devem ser jogados pelos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição deFulvestrantStadafarma
- O princípio ativo é fulvestrante. Cada seringa precarregada (5 ml) contém 250 mg de fulvestrante. Cada ml de solução contém 50 mg de fulvestrante.
- Os demais componentes (excipientes) são etanol (96 %), álcool benzílico, benzoato de benzilo e óleo de rícino refinado.
Aspecto deFulvestrantStadafarmae contenido do envase
Fulvestrant Stadafarma é uma solução viscosa, transparente, de incolora a amarela, em uma seringa precarregada com tampão de bromobutilo, êmbolo de poliestireno e tope de polipropileno que contém 5 ml de solução injetável. Para completar a dose mensal recomendada de 500 mg, devem ser administradas duas seringas.
Fulvestrant Stadafarma apresenta 3 formatos, bem um envase que contém 1 seringa de vidro precarregada, um envase que contém 2 seringas de vidro precarregadas ou bem um envase que contém 6 seringas de vidro precarregadas. São proporcionadas, além disso, agulhas com sistema de segurança (“BD SafetyGlide”) para sua conexão ao corpo de cada seringa.
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Titular da autorização de comercialização e responsável pela fabricação
Titular da autorização de comercialização
Laboratório STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
Espanha
Responsável pela fabricação
STADA Arzneimittel AG
Stadastrasse 2-18
61118 Bad Vilbel
Alemanha
ou
KeVaRo GROUP Ltd.
9 Tzaritza Elenora Str., office 23
Sófia 1618
Bulgária
ou
Laboratori Fundació Dau
Calle Lletra C de la Zona Franca 12-14,
Polígono Industrial de la Zona Franca de Barcelona,
08040, Barcelona,
Espanha
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Alemanha Fulvestrant AL 250 mg Injektionslösung in einer Fertigspritze
Itália Fulvestrant EG STADA
Hungria Fulmerak 250 mg oldatos injekció eloretöltött fecskendoben
Portugal Fulvestrant ELC
Espanha Fulvestrant STADAFARMA 250 mg solução injetável em seringa precarregada EFG
Data da última revisão deste prospecto:dezembro 2023
A informação detalhada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) (http://www.aemps.gob.es/)
Esta informação está destinada apenas a profissionais do sector sanitário:
Fulvestrante 500 mg (2 x 250 mg/5 ml solução injetável) deve ser administrado empregando duas seringas precarregadas, ver seção 3.
Instruções de administração
Advertência – Não esterilize em autoclave a agulha com sistema de segurança (Aguja Hipodérmica Protegida “BD SafetyGlide”) antes de sua utilização. As mãos devem permanecer por detrás da agulha em todo o momento durante sua utilização e eliminação.
Para cada uma das duas seringas:
- Retire o corpo de vidro da seringa da bandeja e verifique que não está danificado.
- Abra o envase exterior da agulha com sistema de segurança (“SafetyGlide”).
- Antes de sua administração, devem ser inspecionadas visualmente as soluções parenterais quanto ao conteúdo em partículas e à decoloração.
- Sostenha a seringa em posição vertical pela parte estriada (C). Com a outra mão, segure o tampão de rosca (A) e gire-o com cuidado em sentido contrário às agulhas do relógio até que se desprenda e possa retirá-lo (ver a Figura 1).
Figura 1
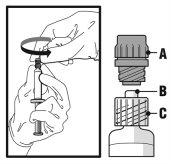
- Retire o tampão de rosca (A) puxando-o reto para cima. Para manter a esterilidade, não toque a ponta da seringa (B) (ver a Figura 2).
Figura 2
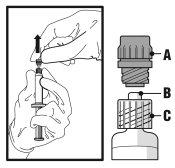
- Acople a agulha com sistema de segurança ao “Luer-Lok” e enrosque-a até que se acople firmemente (ver Figura 3).
- Verifique que a agulha está acoplada ao conector Luer antes de deixar de mantê-la em posição vertical.
Figura 3
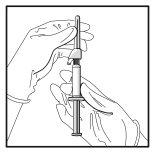
- Tire do capuchão protetor da agulha em linha reta para não danificar o extremo da mesma.
- Leve a seringa carregada ao ponto de administração.
- Retire o capuchão protetor da agulha.
- Elimine o excesso de gás da seringa.
- Proceda à administração por via intramuscular lentamente (de 1 a 2 minutos por injeção) no glúteo (zona glútea). Para maior comodidade do usuário, a posição da agulha com o bisel para cima tem a mesma orientação que o braço da palanca (ver a Figura 4).
Figura 4
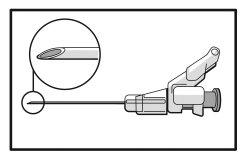
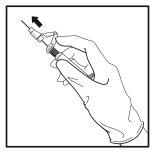
- Após a injeção, dê imediatamente um único toque com o dedo no braço da palanca para ativar o mecanismo de proteção (ver Figura 5).
NOTA: Ative o mecanismo de proteção afastado do seu corpo e dos demais. Escute o clique e confirme visualmente que a ponta da agulha está totalmente protegida.
Figura 5
Eliminação
As seringas precarregadas são sópara um único uso.
Este medicamento pode representar um risco para o meio aquático. Qualquer medicamento não utilizado ou material de desperdício deve ser eliminado conforme as normas locais.

Quanto custa o FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA em Espanha em 2025?
O preço médio do FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA em dezembro de 2025 é de cerca de 408.86 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia408.86 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDAForma farmacêutica: INJETÁVEL, 250 mg/5 mlSubstância ativa: fulvestrantFabricante: Bexal Farmaceutica S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, 250 mgSubstância ativa: fulvestrantFabricante: Ever Valinject GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 250 mgSubstância ativa: fulvestrantFabricante: Astrazeneca AbRequer receita médica
Alternativas a FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA em Polónia
Alternativa a FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA em Ukraine
Médicos online para FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de FULVESTRANT STADAFARMA 250 mg SOLUÇÃO INJETÁVEL EM SERINGA PREENCHIDA – sujeita a avaliação médica e regras locais.












