AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada

Pergunte a um médico sobre a prescrição de AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada

Como usar AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada
Introdução
Prospecto: informação para o paciente
Afultrant 250 mg solução injetável em seringa precarregada EFG
fulvestrante
Leia todo o prospecto atentamente antes de começar a usar este medicamento,pois contém informações importantes para si.
- Conserva este prospecto, pois pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, pois pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Afultrant e para que se utiliza
- O que precisa saber antes de começar a usar Afultrant
- Como usar Afultrant
- Possíveis efeitos adversos
- Conservação de Afultrant
- Conteúdo do envase e informação adicional
1. O que é Afultrant e para que se utiliza
Afultrant contém o princípio ativo fulvestrante, que pertence ao grupo de bloqueantes de estrógeno.
Os estrógenos, um tipo de hormonas sexuais femininas, podem estar em alguns casos implicados no desenvolvimento do cancro da mama.
Afultrant é utilizado:
- em monoterapia, para o tratamento de mulheres pós-menopáusicas com um tipo de cancro da mama chamado cancro da mama com receptor estrogénico positivo, que é localmente avançado ou que se espalhou para outras partes do corpo (metastásico), ou
- em combinação com palbociclib para tratar mulheres com um tipo de cancro da mama chamado cancro da mama com receptor hormonal positivo e cancro da mama com receptor 2 do factor de crescimento epidérmico humano negativo, que está localmente avançado ou que se espalhou para outras partes do corpo (metastásico). As mulheres que não tenham atingido a menopausa também serão tratadas com um medicamento chamado agonista da hormona liberadora da hormona luteinizante (LHRH).
Quando Afultrant for administrado em combinação com palbociclib, é importante que também leia o prospecto de palbociclib. Se tiver alguma dúvida sobre palbociclib, consulte o seu médico.
2. O que precisa saber antes de começar a usar Afultrant
Não useAfultrant:
- se for alérgico a fulvestrante ou a algum dos outros componentes deste medicamento (incluídos na seção 6),
- se estiver grávida ou em período de amamentação,
- se apresentar problemas graves de fígado.
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Afultrant se algo disto se aplicar a si:
- problemas de rim ou fígado,
- contagem baixa de plaquetas (que ajudam à coagulação do sangue) ou alterações hemorrágicas,
- problemas prévios de coágulos sanguíneos,
- osteoporose (perda de densidade óssea),
- alcoolismo.
Crianças e adolescentes
Afultrant não é indicado em crianças e adolescentes menores de 18 anos.
Outros medicamentos eAfultrant
Informa o seu médico ou farmacêutico se estiver a tomar, tomou recentemente ou possa ter que tomar qualquer outro medicamento.
Em particular, deve informar o seu médico se estiver a utilizar anticoagulantes (medicamentos para prevenir os coágulos sanguíneos).
Gravidez e amamentação
Se estiver grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico antes de utilizar este medicamento e durante dois anos após a sua última dose.
Não deve utilizar fulvestrante se estiver grávida. Se puder engravidar, deve utilizar um método anticonceptivo eficaz enquanto estiver em tratamento com fulvestrante.
Não deve amamentar enquanto estiver em tratamento com fulvestrante.
Condução e uso de máquinas
Não se espera que fulvestrante afete a sua capacidade para conduzir ou utilizar máquinas. No entanto, se se sentir cansada após o tratamento, não conduza nem utilize máquinas.
Afultrant contém etanol, álcool benzílico e benzoato de benzilo
Este medicamento contém 1.000 mg de álcool (etanol 96%) por dose, equivalente a 100 mg/ml (10% p/v). A quantidade por dose administrada é equivalente a menos de 24 ml de cerveja ou 10 ml de vinho. A pequena quantidade de álcool que contém este medicamento não produz qualquer efeito perceptível.
Este medicamento contém 1000 mg de álcool benzílico por dose administrada, equivalente a 100 mg/ml.
O álcool benzílico pode provocar reações alérgicas.
O álcool benzílico foi relacionado com o risco de efeitos adversos graves que incluem problemas respiratórios ("síndrome de jadeo") em crianças.
Não administre este medicamento ao seu recém-nascido (até 4 semanas de idade) a menos que tenha sido recomendado pelo seu médico.
Este produto não deve ser utilizado durante mais de uma semana em crianças menores de 3 anos de idade, a menos que tenha sido indicado pelo seu médico ou farmacêutico.
Consulte o seu médico ou farmacêutico se tiver doenças de fígado ou rim. Isto é devido a que se podem acumular no organismo grandes quantidades de álcool benzílico e provocar efeitos adversos (acidose metabólica).
Este medicamento contém 1500 mg de benzoato de benzilo por dose administrada, equivalente a 150 mg/ml.
O benzoato de benzilo pode aumentar o risco de icterícia (coloração amarelada da pele e dos olhos) nos recém-nascidos (até 4 semanas de idade).
3. Como usar Afultrant
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
A dose recomendada é de 500 mg de fulvestrante (duas injeções de 250 mg) administrada uma vez por mês com uma dose adicional de 500 mg administrada 2 semanas após a dose inicial.
O seu médico ou enfermeiro administrará fulvestrante mediante uma injeção intramuscular lenta em cada um dos seus glúteos.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Possíveis efeitos adversos
Assim como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Pode precisar de tratamento médico urgente se experimentar algum dos seguintes efeitos adversos:
- reações alérgicas (hipersensibilidade), incluindo inchaço do rosto, lábios, língua e/ou garganta, que podem ser sintomas de reações anafilácticas,
- tromboembolismo (aumento do risco de coágulos sanguíneos)*,
- inflamação do fígado (hepatite),
- insuficiência hepática.
Informa imediatamente o seu médico, farmacêutico ou enfermeiro se notar algum dos seguintes efeitos adversos:
Efeitos adversos muito frequentes(pode afetar mais de 1 de cada 10 pacientes)
- reações no local da injeção, como dor e/ou inflamação,
- níveis anómalos de enzimas hepáticas (em análise de sangue)*,
- náuseas (sensação de mal-estar),
- fraqueza, cansaço*,
- dor articular e musculoesquelética,
- sofocos,
- erupção cutânea,
- reações alérgicas (hipersensibilidade), incluindo inchaço do rosto, lábios, língua e/ou garganta.
Todos os efeitos adversos restantes:
Efeitos adversos frequentes(pode afetar até 1 de cada 10 pacientes)
- dor de cabeça,
- vómitos, diarreia ou perda de apetite*,
- infecções do trato urinário,
- dor de costas*,
- aumento de bilirrubina (um pigmento da bile produzido pelo fígado),
- tromboembolismo (aumento do risco de coágulos sanguíneos)*,
- níveis diminuídos de plaquetas (trombocitopenia),
- hemorragia vaginal,
- dor lombar que se reflete em um lado da perna (ciática),
- fraqueza súbita, entorpecimento, formigamento ou perda de movimento na sua perna, especialmente em um só lado do corpo, problemas súbitos para caminhar ou de equilíbrio (neuropatia periférica).
Efeitos adversos pouco frequentes(pode afetar até 1 de cada 100 pacientes)
- fluxo vaginal espesso, branco e candidíase (infecção),
- hematoma e hemorragia no local da injeção,
- aumento de gama-GT, uma enzima hepática que se identifica em uma análise de sangue,
- inflamação do fígado (hepatite),
- insuficiência hepática,
- entorpecimento, formigamento e dor,
- reações anafilácticas.
- Inclui efeitos adversos para os quais não se pode avaliar o papel exato de fulvestrante devido à doença subjacente.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Afultrant
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no envase ou nas etiquetas das seringas após a abreviatura CAD/EXP. A data de validade é o último dia do mês que se indica.
Este medicamento não requer condições especiais de conservação.
O seu profissional de saúde será o responsável pela conservação, uso e eliminação corretos de Afultrant.
Não utilize este medicamento se observar que o dispositivo ou o conteúdo está de algum modo deteriorado, como um dano na seringa, a solução não está clara, há partículas em suspensão ou há uma mudança na cor da solução.
Este medicamento pode apresentar um risco para o meio aquático. Os medicamentos não devem ser jogados nos desgues ou na lixeira. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Afultrant
- O princípio ativo é fulvestrante.
Cada seringa pré-carregada contém 250 mg de fulvestrante em 5 ml de solução (50 mg/ml).
- Os excipientes são etanol (96 por cento), álcool benzílico, benzoato de benzilo e óleo de rícino.
Aspecto do produto e conteúdo do envase
Afultrant é uma solução viscosa, transparente, de incolora a amarela em uma seringa pré-carregada.
Afultrant é fornecido em uma ou duas seringas de uso único pré-carregadas. É fornecida uma agulha estéril adicionalmente.
Pode ser que apenas alguns tamanhos de embalagens sejam comercializados.
Título da autorização de comercialização
Bexal Farmacêutica, S.A.
Centro Empresarial Parque Norte
Edifício Carvalho
Rua Serrano Galvache, 56
28033 Madrid
Espanha
Responsável pela fabricação
Lek Pharmaceuticals d.d.
Verovskova 57,
SLO-1526 Liubliana,
Eslovênia
ou
Ebewe Pharma Ges.m.b.H. Nfg.KG
Mondseestrasse 11,
4866 Unterach
Áustria
ou
Fareva Unterach GmbH
Mondseestraße 11
4866 Unterach
Áustria
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Alemanha: Fulvestrante - 1 A Pharma 250 mg Injektionslösung in einer Fertigspritze
Áustria: Fulvestrante Sandoz 250 mg Injektionslösung in einer Fertigspritze
Bélgica: Fulvestrante Sandoz 250 mg oplossing voor injectie in een voorgevulde spuit
Bulgária: Fulvestrante Sandoz 250 mg/5 ml Solution for injection in pre-filled syringe
Croácia: Fulvestrante Sandoz 250 mg otopina za injekciju u napunjenoj štrcaljki
Dinamarca: Fulvestrante Sandoz
Eslováquia: Fulvestrante Sandoz 250 mg, injekcný roztok naplnený v injekcnej striekacke
Eslovênia: Fulvestrante Lek 250 mg raztopina za injiciranje v napolnjeni injekcijski brizgi
Estônia: Fulvestrante Sandoz, 250 mg süstelahus süstlis
Espanha: Afultrant 250 mg solução injetável EFG
Finlândia: Fulvestrante Sandoz 250 mg injektioneste, liuos, esitäytetty ruisku
França: FULVESTRANTE SANDOZ 250 mg, solution injectable en seringue pré-remplie
Holanda: Fulvestrante Sandoz 50 mg/ml, oplossing voor injectie in voorgevulde spuit
Hungria: Fulvestrante Sandoz 250 mgoldatos injekció eloretöltött fecskendoben
Irlanda: Fulvestrante Rowex 250 mg/5 ml solution for injection in pre-filled syringe
Islândia: Fulvestrante Sandoz 250 mg stungulyf, lausn í áfylltri sprautu
Itália: Fulvestrante Sandoz
Lituânia: Fulvestrante Sandoz 250 mg injekcinis tirpalas užpildytame švirkšte
Noruega: Fulvestrante Sandoz 250 mg injeksjonsvæske, oppløsning i ferdigfylt sprøyte
Polônia: Fulvestrante Sandoz, 250 mg/5 ml roztwór w ampulko-strzykawce
Portugal: Fulvestrante Sandoz 250 mg solução injetável em seringa pré-cheia
Reino Unido: Fulvestrante 250 mg solution for injection in pre-filled syringe
República Checa: Fulvestrante Sandoz 250 mg injekcní roztok v predplnené injekcní stríkacce
Romênia: Fulvestrante Sandoz 250 mg solutie injectabila in seringa preumpluta
Suécia: Fulvestrante Sandoz 250 mg injektionsvätska, lösning i förfylld spruta
Data da última revisão deste prospecto:janeiro 2022
A informação detalhada deste medicamento está disponível no site da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) (http://www.aemps.gob.es/)
Esta informação está destinada apenas a profissionais do setor sanitário:
Afultrant 500 mg (2 x 250 mg solução injetável) deve ser administrado usando duas seringas pré-carregadas. Ver seção 3.
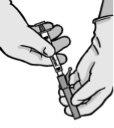
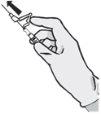
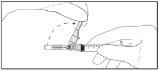
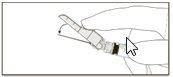
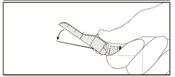
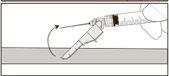
Instruções de administração
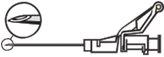
Aviso – Não esterilize em autoclave a agulha com sistema de segurança antes de seu uso. As mãos devem permanecer por trás da agulha em todo momento durante seu uso e eliminação.
As agulhas são fornecidas com sistema de segurança BD SafetyGlide ou Terumo SurGuard
Instruções para as agulhas com sistema de segurança BD SafetyGlide
Para cada uma das duas seringas:
| |
| |
| |
| |
|
|
| |
| |
|
|
|
|
NOTA: Ative longe do seu corpo e dos demais. Escute o clique e confirme visualmente que a ponta da agulha está totalmente protegida.
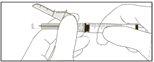
Instruções para as agulhas com sistema de segurança Terumo SurGuard
Para cada uma das duas seringas:
| |
| |
|
|
|
|
| |
| |
| |
|
|
|
|
|
|
A ativação pode ser verificada escutando um ”click” ou de forma táctil e pode ser comprovada visualmente.
Se não estiver seguro de que o protetor de segurança está completamente ativado, repita este passo.
Eliminação
As seringas pré-carregadas são para um únicouso.
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele, será realizada de acordo com a normativa local.

Quanto custa o AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada em Espanha em 2025?
O preço médio do AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada em dezembro de 2025 é de cerca de 408.86 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia408.86 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregadaForma farmacêutica: INJETÁVEL, 250 mgSubstância ativa: fulvestrantFabricante: Ever Valinject GmbhRequer receita médicaForma farmacêutica: INJETÁVEL, 250 mgSubstância ativa: fulvestrantFabricante: Astrazeneca AbRequer receita médicaForma farmacêutica: INJETÁVEL, 250 mgSubstância ativa: fulvestrantFabricante: Reddy Pharma Iberia S.A.Requer receita médica
Alternativas a AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada em Polónia
Alternativa a AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada em Ukraine
Médicos online para AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de AFULTRANT 250 mg Solução Injetável em Seringa Pré-carregada – sujeita a avaliação médica e regras locais.