SANDOSTATIN LAR 20 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE

Cómo usar SANDOSTATIN LAR 20 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
SANDOSTATIN LAR 20 mg polvo y disolvente para suspensión inyectable
octreotida
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Sandostatin LAR y para qué se utiliza
- Qué necesita saber antes de empezar a usar Sandostatin LAR
- Cómo usar Sandostatin LAR
- Posibles efectos adversos
- Conservación de Sandostatin LAR
- Contenido del envase e información adicional
1. Qué es Sandostatin LAR y para qué se utiliza
Sandostatin LAR es un compuesto sintético derivado de la somatostatina. La somatostatina se encuentra normalmente en el cuerpo humano, donde inhibe la liberación de algunas hormonas como la hormona del crecimiento. Las ventajas de Sandostatin LAR respecto a somatostatina son que es más potente y sus efectos son más duraderos.
Sandostatin LAR se utiliza
- para tratar la acromegalia,
La acromegalia es una enfermedad en la que el cuerpo produce demasiada hormona del crecimiento. Normalmente la hormona del crecimiento controla el crecimiento de los tejidos, los órganos y los huesos. Un exceso de hormona del crecimiento supone un aumento en el tamaño de los huesos y los tejidos, especialmente en las manos y los pies. Sandostatin LAR reduce notablemente los síntomas de la acromegalia, que incluyen dolor de cabeza, exceso de sudoración, adormecimiento de las manos y los pies, cansancio y dolor en las articulaciones. En la mayoría de los casos, la sobreproducción de hormona del crecimiento está causada por aumento del tamaño de la glándula pituitaria (adenoma pituitario); el tratamiento con Sandostatin LAR puede reducir el tamaño del adenoma.
Sandostatin LAR se utiliza para tratar personas que sufren acromegalia:
- cuando otros tipos de tratamiento para la acromegalia (cirugía o radioterapia) no son adecuados o no han funcionado correctamente;
- después de la radioterapia, para cubrir el periodo de tiempo intermedio hasta que la radioterapia es completamente eficaz.
- para aliviar los síntomas asociados con la sobreproducción de algunas hormonas específicas y otras sustancias relacionadas en el estómago, intestino o páncreas.
La sobre producción de unas hormonas específicas y de otras sustancias naturales relacionadas puede estar causada por algunas alteraciones raras del estómago, intestino o páncreas. Esto altera el equilibrio hormonal natural y provoca una serie de síntomas como sofocos, diarrea, baja tensión arterial, erupción cutánea y pérdida de peso. El tratamiento con Sandostatin LAR ayuda a controlar estos síntomas.
- para tratar tumores neuroendocrinos localizados en el intestino (p.ej. apéndice, intestino delgado o colon)
Los tumores neuroendocrinos son tumores raros que se pueden encontrar en diferentes partes del cuerpo. Sandostatin LAR también se utiliza para controlar el crecimiento de estos tumores, cuando están localizados en el intestino (p.ej. apéndice, intestino delgado o colon)
- para tratar tumores pituitarios que producen demasiada hormona estimulante del tiroides (TSH).
Un exceso de hormona estimulante del tiroides (TSH) provoca hipertiroidismo. Sandostatin LAR se utiliza para tratar personas con tumores pituitarios que producen demasiada hormona estimulante del tiroides (TSH):
- cuando otros tipos de tratamiento (cirugía o radioterapia) no son adecuados o no han funcionado;
después de la radioterapia, para cubrir el periodo hasta que la radioterapia es completamente efectiva.
2. Qué necesita saber antes de empezar a usar Sandostatin LAR
Siga todas las instrucciones que le dé su médico cuidadosamente. Pueden ser diferentes de la información contenida en este prospecto.
Lea las siguientes indicaciones antes de utilizar Sandostatin LAR.
No use Sandostatin LAR:
- si es alérgico a octreotida o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Sandostatin LAR:
- si sabe que tiene actualmente cálculos en la vesícula biliar, o los ha tenido en el pasado o presenta alguna complicación como fiebre, escalofríos, dolor abdominal, o coloración amarillenta de la piel o los ojos; informe a su médico, pues el uso prolongado de Sandostatin LAR puede provocar la formación de cálculos biliares. Su médico prodría querer controlar su vesícula biliar periódicamente.
- si sabe que tiene diabetes, ya que Sandostatin LAR puede afectar los niveles de azúcar en la sangre. Si es diabético, debe controlar regularmente sus niveles de azúcar.
- si tiene antecedentes de deficiencia de vitamina B12 su médico puede controlar el nivel de B12 periodicamente.
Análisis y controles
Si recibe tratamiento con Sandostatin LAR durante un periodo prolongado de tiempo, su médico puede controlar su función tiroidea periódicamente.
Su médico controlará la función de su hígado.
Su médico podrá comprobar el funcionamiento de sus enzimas pancreáticas.
Niños
Existe poca experiencia con el uso de Sandostatin LAR en niños.
Uso de Sandostatin LAR con otros medicamentos
Comunique a su médico o farmacéutico si está tomando, ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
Normalmente puede continuar tomando otros medicamentos mientes esté en tratamiento con Sandostatin LAR. Sin embargo, se han notificado que algunos medicamentos como cimetidina, ciclosporina, bromocriptina, quinidina y terfenadina se ven afectados por Sandostatin LAR.
Si está tomando un medicamento para controlar la presión arterial (p.ej. un beta bloqueante o un antagonista de los canales del calcio) o un agente para controlar el equilibrio de líquidos y electrolitos, su médico puede necesitar ajustar la dosis.
Si es diabético, su médico puede necesitar ajustar su dosis de insulina.
Si va a recibir tratamiento con lutecio (177Lu) oxodotreotida, un radiofármaco, su médico puede interrumpir y/o adaptar el tratamiento con Sandostatin LAR durante un periodo corto de tiempo.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Sólo se debe utilizar Sandostatin LAR durante el embarazo si es estrictamente necesario.
Las mujeres en edad fértil deben utilizar un método anticonceptivo eficaz durante el tratamiento.
No debe dar lactancia durante el tratamiento con Sandostatin LAR. Se desconoce si Sandostatin LAR pasa a la leche materna.
Conducción y uso de máquinas
Sandostatin LAR no tiene efectos o éstos son insignificantes sobre la capacidad de conducir o utilizar máquinas. Sin embargo algunos de estos efectos adversos que puede sufrir durante el tratamiento con Sandostatin LAR, como dolor de cabeza y cansancio, pueden reducir su capacidad para conducir y utilizar máquinas de forma segura.
Sandostatin contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por vial; esto es, esencialmente “exento de sodio”.
3. Cómo usar Sandostatin LAR
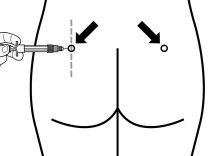
Sandostatin LAR se debe administrar siempre como una inyección en el músculo de los glúteos. Con la administración repetida, se debe utilizar el glúteo derecho e izquierdo alternativamente.
Si usa más Sandostatin LAR del que debe
No se han notificado reacciones adversas que suponen una amenaza vital después de una sobredosis con Sandostatin LAR.
Los síntomas de sobredosis son: sofocos, micciones frecuentes, cansancio, depresión, ansiedad y falta de concentración.
Si piensa que ha sufrido una sobredosis y presenta alguno de estos síntomas, informe a su médico inmediatamente. También puede llamar al Servicio de Información Toxicológica, Tel. 91 562 0420.
Si olvidó usar Sandostatin LAR
Si ha olvidado su inyección, se recomienda que se la administren tan pronto como se acuerde, y después continuar con la pauta habitual. No va a provocarle ningún daño el hecho de recibir una dosis unos días más tarde, pero pueden reaparecer temporalmente los síntomas hasta que vuelva a la pauta habitual de tratamiento.
Si interrumpe el tratamiento con Sandostatin LAR
Si interrumpe su tratamiento con Sandostatin LAR pueden reaparecer sus síntomas. Por tanto, no interrumpa el tratamiento con Sandostatin LAR a menos que se lo indique su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, enfermero o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunas reacciones adversas podrían ser graves. Informe a su médico inmediatamente si sufre alguno de los siguientes:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Cálculos biliares, que conllevan dolor de espalda repentino.
- Demasiado azúcar en la sangre.
Frecuentes(pueden afectar hasta 1 de cada 10 personas:
- Disminución de la actividad de la glándula tiroidea (hipotiroidismo) que causa cambios en el ritmo del corazón, el apetito o el peso; cansancio, sensación de frío, o hinchazón en la parte de delante del cuello.
- Cambios en los análisis de la función tiroidea.
- Inflamación de la vesícula biliar (colecistitis); los síntomas pueden incluir dolor en la parte superior derecha del abdomen, fiebre, náuseas, coloración amarilla de la piel y los ojos (ictericia).
- Muy poco azúcar en la sangre.
- Alteración de la tolerancia a la glucosa.
- Latido del corazón lento.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- Sed, baja eliminación de orina, color oscuro en la orina, piel seca enrojecida.
- Latido del corazón rápido.
Otras reacciones adversas graves
- Reacciones de hipersensibilidad (alérgicas) incluyendo urticaria en la piel.
- Un tipo de reacción alérgica (anafilaxis) que puede causar dificultad para tragar o respirar, hinchazón y hormigueo, posiblemente con una disminución de la tensión arterial con mareo o pérdida de conciencia.
- Una inflamación de la glándula del páncreas (pancreatitis); los síntomas pueden incluir dolor repentino en la parte superior del abdomen, náuseas, vómitos, diarrea.
- Inflamación del hígado (hepatitis); síntomas que pueden incluir color amarillento de la piel y los ojos (ictericia), náuseas, vómitos, pérdida de apetito, sensación general de malestar, picor, orina ligeramente coloreada.
- Latido del corazón irregular.
- Nivel bajo en el recuento de plaquetas en la sangre; esto puede suponer un aumento en el sangrado o en la aparición de moratones.
Informe a su médico inmediatamente si nota alguno de las reacciones adversas anteriores.
Otros efectos adversos:
Informe a su médico, farmacéutico o enfermero si nota alguno de los efectos adversos enumerados a continuación. Normalmente son leves y tienden a desaparecer al avanzar el tratamiento.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- Diarrea.
- Dolor abdominal.
- Náuseas.
- Estreñimiento.
- Flatulencia (gases).
- Dolor de cabeza.
- Dolor local en el lugar de inyección.
Frecuentes(puede afectar hasta 1 de cada 10 personas):
- Molestia en el estómago después de comer (dispepsia).
- Vómitos.
- Sensación de tener el estómago lleno.
- Heces grasas.
- Heces líquidas.
- Cambio de color de las heces.
- Mareo.
- Pérdida de apetito.
- Cambios en los análisis sobre la función del hígado.
- Pérdida de pelo.
- Dificultad para respirar.
- Debilidad.
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, enfermero o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: http://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Sandostatin LAR
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en el embalaje original para protegerlo de la luz.
Conservar en nevera (entre 2ºC y 8ºC). No congelar.
Sandostatin LAR se puede conservar por debajo de 25ºC el día de la inyección.
No se debe conservar Sandostatin LAR después de la reconstitución (se debe utilizar inmediatamente).
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa presencia de partículas o un cambio de color.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Sandostatin LAR
- El principio activo es octreotida.
Un vial contiene 20 mg de octreotida (como octreotida acetato).
- Los demás componentes son:
En el polvo (vial): poli(DL-láctido-co-glicólido), manitol (E421).
En el disolvente (jeringa precargada): carboximetilcelulosa sódica, manitol (E421), poloxamer 188, agua para preparaciones inyectables
Aspecto de Sandostatin LAR y contenido del envase
Envases unitarios que contienen un vial de vidrio de 6 ml con tapón de goma (bromobutilo), sellado con una lengüeta de aluminio, que contienen el polvo para suspensión inyectable y una jeringa precargada de vidrio incoloro de 3 ml con un tapón frontal y un tapón de émbolo (goma clorobutilo) con 2 ml de disolvente, envasados conjuntamente en una bandeja blister sellada con un adaptador al vial y una aguja de inyección de seguridad.
Envases múltiples de tres envases unitarios, que cada uno contiene: un vial de vidrio de 6 ml con tapón de goma (bromobutilo), sellado con una lengüeta de aluminio, que contiene el polvo para suspensión inyectable y una jeringa precargada de vidrio incoloro de 3 ml con un tapón frontal y un tapón de émbolo (goma clorobutilo) con 2 ml de disolvente, envasados conjuntamente en una bandeja blister sellada con un adaptador al vial y una aguja de inyección de seguridad.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Novartis Farmacéutica, S.A.
Gran Vía de les Corts Catalanes, 764
08013 Barcelona
Responsable de la fabricación
Novartis Farmacéutica, S.A.
Gran Vía de les Corts Catalanes, 764
08013 Barcelona
Novartis Pharma GmbH
Jakov-Lind-Straße 5, Top 3.05 1020 Wien Austria
Novartis Pharma NV
Medialaan 40/Bus 1, 1800 Vilvoorde, (Bélgica).
Novartis Healthcare A/S
Edvard Thomsens vej 14, DK-2300 Copenhagen S, (Dinamarca).
Novartis Finland Oy
Metsänneidonkuja 10, 02130 Espoo, (Finlandia)
Novartis Pharma SAS
8-10 rue Henri Sainte-Claire Deville, 92500 Rueil-Malmaison (Francia)
Novartis Pharma GmbH
Roonstrasse 25, 90429 Nürnberg, (Alemania).
Novartis Pharma GmbH
Sophie-Germain-Strasse 10, 90443 Nürnberg (Alemania).
Novartis Farma-Produtos Farmacéuticos, SA
Avenida Professor Doutor Cavaco Silva nº10E, Taguspark, 2740-255 Porto Salvo, (Portugal).
Novartis Farma S.p.A.
Via Provinciale Schito 131, 80058 Torre Annunziata, NA , (Italia).
Novartis Farma S.p.A.
Viale Luigi Sturzo 43, 20154 - Milan (MI) (Italia)
Novartis Sverige AB
Torshamnsgatan 48, 164 40 Kista (Suecia)
Novartis (Hellas) S.A.
12th km National Road Athinon-Lamia 14451 Metamorphosis Attiki, (Grecia).
Novartis Pharma B.V.
Haaksbergweg 16, 1101 BX Amsterdam (Holanda).
Novartis Poland Sp. z o.o.
15 Marynarska Street, 02-674 Varsovia (Polonia).
Novartis Hungária Kft.
Vasút u.13, 2040 Budaörs (Hungría).
Abbot Biologicals B.V.
Veerweg, 12. 8121 AA Olst (Holanda)
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria, Bulgaria, Croacia, Chipre, República Checa, Dinamarca, Estonia, Finlandia, Alemania, Grecia, Hungría, Islandia, Irlanda, Letonia, Lituania, Malta, Noruega, Polonia, Rumanía, Eslovaquia, Eslovenia, España, Suecia | Sandostatin LAR |
Bélgica, Luxemburgo, Holanda | Sandostatine LAR |
Italia, Portugal | Sandostatina LAR |
Francia | Sandostatine L.P. |
Fecha de la última revisión de este prospecto:12/2023
Otras fuentes de información
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
Esta información está destinada únicamente a profesionales del sector sanitario:
Cuánto Sandostatin LAR se debe utilizar
Acromegalia
Se recomienda iniciar el tratamiento con la administración de 20 mg de Sandostatin LAR a intervalos de 4 semanas durante 3 meses. Los pacientes en tratamiento con Sandostatin s.c. pueden iniciar el tratamiento con Sandostatin LAR el día después de la última dosis de Sandostatin s.c.. El ajuste de dosis subsiguiente se debe basar en las concentraciones séricas de la hormona del crecimiento (GH) y el factor de crecimiento 1 de tipo insulina/somatomedina C (IGF-1) y los síntomas clínicos.
Para los pacientes en los que no están controlados completamente los síntomas clínicos y los parámetros bioquímicos (GH; IGF-1) en un periodo de 3 meses (concentraciones de GH todavía por encima de 2,5 microgramos/L), se puede aumentar la dosis a 30 mg cada 4 semanas. Si después de 3 meses, no están controlados adecuadamente GH, IGF-1, y/o los síntomas a la dosis de 30 mg, la dosis se puede aumentar a 40 mg cada 4 semanas.
Para pacientes con concentraciones de GH que se encuentran de forma constante por debajo de 1 microgramo/L, y con concentraciones séricas de IGF‑1 normalizadas, y en los cuales la mayoría de los signos/síntomas reversibles de acromegalia han desaparecido después de 3 meses de tratamiento con 20 mg, se pueden administrar 10 mg de Sandostatin LAR cada 4 semanas. Sin embargo, especialmente en este grupo de pacientes, se recomienda controlar estrechamente las concentraciones séricas de GH e IGF‑1, y los signos/síntomas a esta dosis baja de Sandostatin LAR.
Para pacientes que están tratados con una dosis estable de Sandostatin LAR, la valoración de GH e IGF‑1 se debe realizar cada 6 meses.
Tumores endocrinos gastroenteropancreaticos
- Tratamiento de pacientes con síntomas asociados con tumores neuroendocrinos gastroenteropancreaticos funcionales
Se recomienda iniciar el tratamiento con la administración de 20 mg de Sandostatin LAR a intervalos de 4 semanas. Los pacientes en tratamiento con Sandostatin s.c. deben continuar a la dosis previamente efectiva durante 2 semanas después de la primera inyección de Sandostatin LAR.
Para pacientes en los que los síntomas y los marcadores biológicos están bien controlados después de 3 meses de tratamiento, se puede reducir la dosis a 10 mg de Sandostatin LAR cada 4 semanas.
Para pacientes en los que los síntomas están sólo parcialmente controlados después de 3 meses de tratamiento, se puede aumentar la dosis a 30 mg de Sandostatin LAR cada 4 semanas.
Para los días en que pueden aumentar los síntomas asociados con tumores gastroenteropancreáticos durante el tratamiento con Sandostatin LAR, se recomienda la administración adicional de Sandostatin s.c. a la dosis utilizada antes del tratamiento con Sandostatin LAR. Esto puede aparecer principalmente en los 2 primeros meses de tratamiento hasta que se alcancen las concentraciones terapéuticas de octreotida.
- Tratamiento de pacientes con Tumores Neuroendocrinos avanzados del intestino o de origen desconocido donde se ha excluido los lugares de origen que no sean del intestino
La dosis recomendada de Sandostatin LAR es de 30 mg administrado cada 4 semanas. El tratamiento con Sandostatin LAR para controlar el tumor se debe continuar en ausencia de progresión del tumor.
Tratamiento de adenomas secretores de TSH
El tratamiento con Sandostatin LAR se debe iniciar a la dosis de 20 mg a intervalos de 4 semanas durante
3 meses antes de considerar un ajuste de dosis. Después la dosis se ajusta en base de la TSH y de la respuesta de la hormona tiroidea.
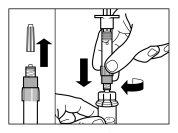
Instrucciones para la preparación y la inyección intramuscular de Sandostatin LAR.
SÓLO PARA INYECCIÓN INTRAMUSCULAR
Componentes del kit de inyección:
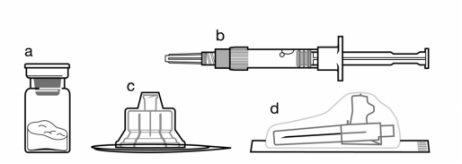
- Un vial que contiene el polvo de Sandostatin LAR
- Una jeringa precargada que contiene el disolvente para la reconstitución
- Un adaptador al vial para la reconstitución del medicamento
- Una aguja de inyección de seguridad
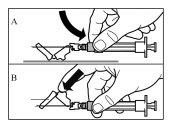
Siga cuidadosamente las instrucciones que se indican a continuación para asegurar la reconstitución de Sandostatin LAR antes de la inyección intramuscular profunda
Hay tres pasos críticos en el proceso de reconstitución de Sandostatin LAR. Si no se realizan correctamente, podría suponer que no se administra el medicamento de forma adecuada.
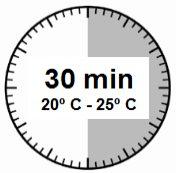
- El kit de inyección debe alcanzar la temperatura ambiente. Sacar el kit de inyección de la nevera y dejar que el kit alcance la temperatura ambiente durante un mínimo de 30 minutos antes de la reconstitución, pero sin que se superen las 24 h.
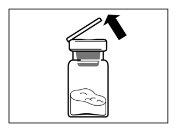
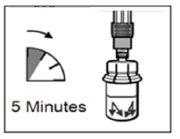
- Después de añadir la solución del diluyente, dejar reposar el vial durante 5 minutos para asegurar que el polvo queda completamente saturado.
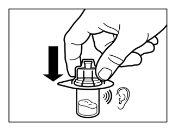
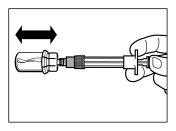
- Después de la saturación, agitar el vial moderadamenteen dirección horizontal durante un mínimo de 30 segundos hasta que se forme una suspensión uniforme. La suspensión de Sandostatin LAR sólo se debe preparar inmediatamenteantes de la administración.
Sandostatin LAR debe ser administrado sólo por un profesional sanitario experto.
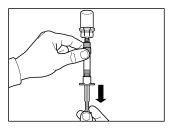
| PASO 1
ATENCIÓN: es esencial iniciar el proceso de reconstitución sólo después de que el kit de inyección haya alcanzado la temperatura ambiente. Dejar que el kit alcance la temperatura ambiente durante un mínimo de 30 minutos antes de la reconstitución pero no exceder las 24 h. Nota: El Kit de inyección puede refrigerarse de nuevo, en caso necesario. |
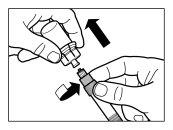
| PASO 2
|
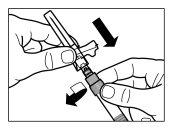
| PASO 3
|
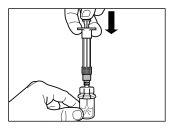
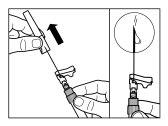
| |
| PASO 4 ATENCIÓN: Es esencial dejar reposar el vial durante 5 minutospara asegurar que el disolvente ha saturado completamente el polvo. Nota: es normal que el émbolo se desplace hacia arriba ya que puede haber una sobrepresión en el vial.
|
| PASO 5
ATENCIÓN:Mantener el émbolo presionado y agitar el vial moderadamenteen sentido horizontal duranteun mínimo de30 segundospara que el polvo esté completamente suspendido en el disolvente (suspensión uniforme de consistencia lechosa). Repetir la agitación moderada durante otros 30 segundos más si el polvo no está completamente suspendido. |
| PASO 6
|
| |
| PASO 7
|
| PASO 8
|
| Paso 9
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SANDOSTATIN LAR 20 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLEForma farmacéutica: INYECTABLE, Octreotida 100 microgramos/mlPrincipio activo: OctreotidaFabricante: Gp Pharm S.A.Requiere recetaForma farmacéutica: INYECTABLE, Octreotida 500 microgramos/mlPrincipio activo: OctreotidaFabricante: Gp Pharm S.A.Requiere recetaForma farmacéutica: INYECTABLE, 200 microgramos/mlPrincipio activo: OctreotidaFabricante: Gp Pharm S.A.Requiere receta
Médicos online para SANDOSTATIN LAR 20 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SANDOSTATIN LAR 20 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes