MENOPUR 600 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar MENOPUR 600 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
MENOPUR 600 Unidades Internacionales polvo y disolvente para solución inyectable
Menotropina altamente purificada
Lea todo el prospecto detenidamente antes de empezar a tomar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque presenten los mismos síntomas de enfermedad que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, o farmacéutico, incluso si se trata de efecto adverso que no aparecen en este prospecto ( ver sección 4).
Contenido del prospecto
- Qué es Menopur y para qué se utiliza
- Qué necesita saber antes de usar Menopur
- Cómo usar Menopur
- Posibles efectos adversos
- Conservación de Menopur
- Contenido del envase e información adicional
1. Qué es Menopur y para qué se utiliza
Menopur contiene menotropina (también llamada gonadotropina menopáusica humana o hMG-HP). Es un extracto altamente purificado de la orina de mujeres menopáusicas, y contiene a su vez dos hormonas llamadas hormona folículo estimulante (FSH) y hormona luteinizante (LH). La FSH y LH están presentes tanto en hombres como en mujeres, y ayudan a los órganos reproductores a funcionar normalmente.
Menopur está indicado para el tratamiento de esterilidad en las siguientes situaciones:
- Mujeres que no pueden quedarse embarazadas porque sus ovarios no producen óvulos (incluyendo síndrome de ovario poliquístico). Menopur se usa en mujeres que han sido tratadas con un medicamento llamado citrato de clomifeno para tratar su infertilidad, pero este medicamento no les ha causado efecto.
- Mujeres en programas de reproducción asistida (TRA) (incluyendo fecundación in vitro/ transferencia embrionaria [FIV/TE], transferencia intratubárica de gametos (GIFT), inyección intracitoplasmática de espermatozoides (ICSI)). Menopur ayuda a los ovarios a desarrollar muchos sacos de óvulos (folículos) donde un óvulo pueda desarrollarse (desarrollo folicular múltiple).
- Esterilidad en hombres con hipogonadismo hipo o normogonadotrópico: en combinación con gonadotropina
coriónica humana para estimular la espermatogénesis.
2. Qué necesita saber antes de usar Menopur
Antes de comenzar el tratamiento con Menopur, usted y su pareja deben ser evaluados por un médico para ver las causas del problema de infertilidad. En particular, se le deberán controlar las siguientes enfermedades para que puedan administrarle el tratamiento más adecuado:
- Mal funcionamiento de la glándula tiroides y de la corteza de las glándulas suprarrenales
- Altos niveles de una hormona llamada prolactina (hiperprolactinemia)
- Tumores en la hipófisis (una glándula localizada en la base del cerebro)
- Tumores en el hipotálamo (un área localizada bajo la parte del cerebro llamada tálamo).
Si sabe que padece de alguna de las enfermedades listadas arriba, por favor comuníqueselo a su médico antes de comenzar el tratamiento con Menopur.
No utilice Menopur si:
- es alérgico (hipersensible) a la menotropina o a cualquiera de los demás componentes de Menopur incluidos en la sección 6.
En mujeres:
- si tiene tumor en el útero, ovarios, mamas, o algunas partes del cerebro como el hipotálamo-hipófisis
- presenta aumento de los ovarios o quistes no provocados por el síndrome del ovario poliquístico
- si tiene malformaciones en los órganos sexuales o matriz (útero)
- presenta sangrado vaginal de causa desconocida
- si tiene fibromas (tumores benignos) en el útero (matriz)
- si está embaraza o en periodo de lactancia
- si tiene menopausia precoz.
En hombres:
- Si tiene carcinoma de próstata
- Si tiene tumor en los testículos.
Advertencias y precauciones
Si tiene:
- Dolor en el abdomen
- Hinchazón del abdomen
- Náuseas
- Vómitos
- Diarrea
- Ganancia de peso
- Dificultad para respirar
- Disminución de la orina.
Consulte directamente con su médico, incluso si los síntomas se desarrollan algunos días después de que se haya administrado la última inyección. Estos pueden ser signos de altos niveles de actividad en los ovarios que pueden convertirse en graves.
Si estos síntomas se vuelven graves, el tratamiento de infertilidad debe retirarse y debe recibir tratamiento en un hospital.
Manteniendo la dosis recomendada y un seguimiento cuidadoso del tratamiento se reducirán las opciones de padecer estos síntomas.
Si deja de usarMenopur puede experimentar aún estos síntomas. Por favor contacte con su médico inmediatamente si sufre cualquiera de estos síntomas.
Mientras esté en tratamiento con este medicamento, su médico normalmente le mandará ecografíasy algunas veces análisis de sangrepara monitorizar su respuesta al tratamiento.
Cuando se está tratado con hormonas como Menopur se puede incrementar el riesgo de:
- Embarazo ectópico (embarazo fuera de la matriz) si tiene historial de enfermedad de trompa de falopio
- Aborto
- Embarazo múltiple (gemelos, trillizos, etc.)
- Malformaciones congénitas (defectos físicos presentados en el bebé al nacer).
Algunas mujeres que han sido tratadas con varios medicamentos por infertilidad han desarrollado tumores en los ovarios y otros órganos reproductores. No se conoce aún si el tratamiento con hormonas como este medicamento causan estos problemas.
Es más probable que se formen coágulos dentro de los vasos sanguíneos ( venas o arterias) en mujeres embarazadas. El tratamiento de infertilidad puede incrementar la probabilidad de que esto ocurra, especialmente si usted tiene sobrepeso o enfermedad de coagulación de la sangre conocida (trombofilia) o si usted o algún familiar (consanguíneo) ha tenido problemas de coagulación. Informe a su médico si piensa que es aplicable a usted.
Uso en niños
Menopur no tiene indicaciones adecuadas para su uso en niños.
Uso de Menopur con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
No se han realizado estudios de interacciones de otros medicamentos con Menopur en humanos.
Cuando se tratan hombres no fértiles, se puede administrar al mismo tiempo la menotropina y la gonadotropina coriónica humana.
El citrato de clomifeno es otro medicamento usado en el tratamiento de infertilidad. Si Menopur se usa al mismo tiempo que citrato de clomifeno el efecto en los ovarios se puede incrementar.
Menopur puede usarse al mismo tiempo que Bravelle (otro medicamento utilizado para el tratamiento de la infertilidad). Por favor ver sección 3 “Cómo usar Menopur”.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, o cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Menopur no está indicado en ningún caso para tratar mujeres embarazadas ó en periodo de lactancia.
Conducción y uso de máquinas
Es muy raro que Menopur afecte a la capacidad de conducir y utilizar máquinas.
Información importante sobre alguno de los componentes de Menopur:
Menopur contiene menos de 1 mmol de cloruro sódico (23 mg) por dosis, por tanto está esencialmente libre de sodio.
3. Cómo usar Menopur
Siga exactamente las instrucciones de administración de Menopur indicadas por su médico.
En caso de duda, consulte de nuevo a su médico o farmacéutico.
- Mujeres que no ovulan (no producen óvulos):
El tratamiento comenzará dentro de los 7 días iniciales del ciclo menstrual (día 1 es el primer día del periodo). El tratamiento deberá administrarse cada día durante al menos 7 días.
La dosis inicial es normalmente 75-150 UI diarias. Esta dosis puede incrementarse de acuerdo a su respuesta al tratamiento hasta un máximo de 225 UI por día. Una dosis individual deberá administrarse al menos 7 días antes de ajustarse la dosis por el médico. El incremento de dosis recomendada es 37,5 UI por ajuste (y no más de 75 UI). El ciclo de tratamiento se deberá abandonar si no responde adecuadamente después de 4 semanas.
Cuando se obtenga una respuesta óptima se administrará una inyección única de otra hormona llamada gonadotropina coriónica humana (hCG), de 5.000 a 10.000 UI, 1 día después de la última dosis de Menopur. Se recomienda tener coitos el mismo día de la administración de hCG y el día siguiente. Se puede realizar inseminación intrauterina alternativamente (inyección de esperma directamente en la matriz). Su médico deberá seguir su progreso muy de cerca durante al menos 2 semanas después de la administración de hCG.
Su médico seguirá el efecto del tratamiento de Menopur. Dependiendo del progreso, su médico decidirá interrumpir el tratamiento con Menopur y no administrarle la inyección de hCG. En este caso, debe utilizar un método anticonceptivo (preservativo) o no tener relaciones sexuales hasta que comience el próximo periodo.
ii. Mujeres en programas de reproducción asistida:
Si usted está también recibiendo tratamiento con agonistas de GnRH (un medicamento que ayuda a la hormona llamada Hormona Liberadora de Gonadotropina (GnRH) a funcionar), el tratamiento con Menopur deberá comenzar aproximadamente 2 semanas después de iniciar el tratamiento agonista de GnRH.
Si usted está también recibiendo tratamiento con antagonistas de GnRH, el tratamiento con Menopur deberá comenzar el día 2 ó 3 del ciclo menstrual (día 1 es el primer día del periodo).
Menopur se administrará diariamente durante al menos 5 días. La dosis inicial recomendada de Menopur es 150-225 Unidades Internacionales. Esta dosis puede incrementarse de acuerdo con su respuesta al tratamiento hasta un máximo de 450 Unidades Internacionales al día. La dosis no deberá incrementarse más de 150 UI por ajuste. Normalmente el tratamiento no se recomienda más de 20 días.
Si hay suficientes sacos de óvulos, se administrará una inyección única de un medicamento llamado gonadotropina coriónica humana (hCG) a una dosis de hasta 10.000 UI para inducir la ovulación (liberación de un óvulo).
Su médico deberá seguir su progreso muy de cerca durante al menos 2 semanas después de la administración de hCG.
Su médico seguirá el efecto del tratamiento de Menopur. Dependiendo del progreso, su médico decidirá interrumpir el tratamiento con Menopur y no administrarle la inyección de hCG. En este caso, debe utilizar un método anticonceptivo (preservativo) o no tener relaciones sexuales hasta que comience el próximo periodo.
Esterilidad en hombres:
Inicialmente, se administran entre 1.000 y 3.000 UI de gonadotropina coriónica humana, 3 veces a la semana, hasta alcanzar un nivel sérico de testosterona normal.
Después se administra una dosis de Menopur de 75-150 UI (1-2 viales) 3 veces a la semana, por vía intramuscular (IM), durante varios meses.
Uso en niños
Menopur no tiene indicaciones adecuadas para su uso en niños.
INSTRUCCIONES DE USO:
Si su médico le dice que se inyecte Menopur usted mismo, deberá seguir cualquier indicación que le
proporcione.
La primera inyección de este medicamento deberá administrarse bajo la supervisión de un médico o enfermera.
Menopur se proporciona como un polvo en un vial y se debe disolver con una jeringa con disolvente antes de su inyección. El líquido que debe utilizar para diluir Menopur, se proporciona en una jeringa precargada.
Menopur 600 UI debe reconstituirse con una jeringa precargada con el disolvente antes de su uso.
Tras disolver el polvo con el disolvente el vial contiene medicación para varios días de tratamiento, por tanto, debe estar seguro que solo extrae la cantidad de medicación que le ha prescrito el médico.
Su médico le ha prescrito una dosis de Menopur en UI (unidades). Deberá usar una de las 9 jeringas de administración graduadas en UI (unidades) FSH/LH proporcionadas.
Siga los siguientes pasos:
|
|
|
|
|
|
1 | 2 | 3 | 4 |
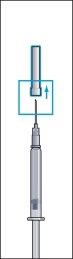
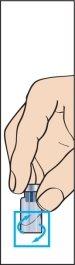
- Retire la capucha protectora del vial de polvo y el tapón de goma de la jeringa precargada con el disolvente (imagen 1).
- Ajuste firmemente la aguja larga y delgada (aguja de reconstitución) a la jeringa precargada con el disolvente y retire la capucha protectora (imagen 2).
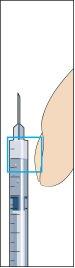
- Inserte la aguja verticalmente a través del centro del tapón de goma del vial del polvo e inyecte lentamente todo el líquidopara evitar la formación de burbujas (imagen 3).
4 Cuando se añade el disolvente se crea una ligera sobrepresión en el vial. Por lo tanto, suelte el émbolo de la jeringa para que se levante por sí mismo durante unos 10 segundos. Esto eliminará el exceso de presión en el vial (imagen 4).
Retire la jeringa y la aguja de reconstitución.
|
|
|
|
|
5 | 6 | 7 | 8 |
- El polvo se disolverá rápidamente (en 2 minutos) formando una solución transparente. Aunque esto normalmente sucede cuando se han añadido sólo unas pocas gotas de disolvente, debe añadirse la cantidad total del disolvente. Para ayudar a la disolución del polvo, mueva el vial suavemente (imagen 5). No agitar, ya que puede causar la formación de burbujas de aire.
No debe utilizarla solución reconstituida si contiene partículas o si no está transparente.
El vial de polvo ahora está disuelto con una jeringa de disolvente y listo para utilizarse.
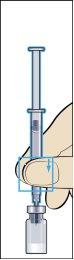
- Coja la jeringa de administración con la aguja prefijada e insertar la aguja verticalmente en el centro del vial. La jeringa de administración ya contiene una pequeña cantidad de aire que se debe inyectar en el vial por encima del líquido. Ponga el vial boca abajo y retire la dosis prescrita de Menopur en la jeringa de administración para inyección (imagen 6).
RECUERDE: debido a que el vial contiene medicación para varios días de tratamiento, debe asegurarse que sólo retira la cantidad de medicación que le ha prescrito el médico.
Si se le ha prescrito Bravelle al mismo tiempo que Menopur, puede mezclar los dos medicamentos reconstituyendo Menopur e inyecte la dosis prescrita de Menopur en la solución reconstituida de Bravelle. Extraiga la solución mezclada, de esta forma puede inyectarse los dos medicamentos conjuntamente y no por separado.
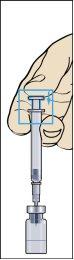
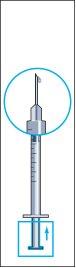
- Retire la jeringa del vial y extraiga una pequeña cantidad de aire de la jeringa (imagen 7).
- Golpee suavemente la jeringuilla de administración de modo que todas las burbujas de aire se quede en la parte superior (imagen 8). Empuje cuidadosamente todo el aire hasta que la primera gota de líquido de la solución salga de la aguja.
Su médico o enfermera le dirá donde inyectarse (por ejemplo en parte frontal del muslo, abdomen etc.).
Antes de la inyección, limpie la piel del lugar de la inyección con un hisopo con alcohol.
|
9 |
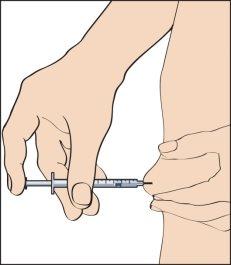
- Para inyectar, pellizque la piel para producir un pliegue, e inserte la aguja en un movimiento rápido a 90 grados del cuerpo. Presione el émbolo para inyectar la disolución (imagen 9), y después retire la jeringa.
Tras retirar la jeringa, aplique presión al lugar de inyección para contener cualquier sangrado. Un masaje cuidadoso en el lugar de inyección ayudará a dispersar la disolución bajo la piel.
No tirar el material usado en la basura, deberá desecharse correctamente.
- Para las siguientes inyecciones con la solución reconstituida de Menopur, repita los pasos 6 a 9.
Si usa más Menopur del que debiera.En caso de sobredosis ó si se traga la solución por accidente, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad utilizada.
Si olvidó usar Menopur,no use una dosis doble para compensar las dosis olvidadas. Por favor dígaselo a su médico o farmacéutico.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos Menopur puede producir efectos adversos, aunque no todas las personas lo sufran.
El tratamiento con Menopur puede causar altos niveles de actividad en los ovariosque pueden dar lugar a una enfermedad llamada Síndrome del Hiperestimulación Ovárica (SHO), especialmente en mujeres con ovarios poliquísticos.Los síntomas incluyen: distensión y molestia en el abdomen, náusea, vómito, diarrea, ganancia de peso.En algunos casos graves de SHO se han notificado como complicaciones raras la acumulación de fluido en el abdomen, pelvis y/o en la cavidad pleural, dificultad para respirar y disminución de la orina.
formación de coágulos de sangre en los vasos sanguíneos (tromboembolismo) y torsión en los ovarios. Si experimenta cualquiera de estos síntomas contacte inmediatamente con su médico, incluso si se desarrollan algunos días después de la administración de la última inyección.
Pueden producirse reacciones alérgicas (hipersensibilidad), cuando se usa este medicamento. Los síntomas de estas reacciones pueden incluir: erupción, picor, hinchazón de la garganta y dificultad para respirar.Si experimenta cualquiera de estos síntomas contacte inmediatamente con su médico.
Los siguientes efectos adversos frecuentesafectan entre 1 y 10 de cada 100 pacientes tratados:
- Dolor abdominal
- Dolor de cabeza
- Nauseas
- Hinchazón (plenitud) abdominal
- Dolor pélvico
- Hiperestimulación de los ovarios que dan como resultado altos niveles de actividad (Síndrome de Hiperestimulación Ovárica)
- Reacciones locales en el lugar de inyección (como dolor, enrojecimiento, contusión, hinchazón y/o irritación).
Los siguientes efectos adversos poco frecuentesafectan entre 1 y 10 de cada 1.000 pacientes tratados:
- Vómitos
- Molestia en el abdomen
- Diarrea
- Fatiga
- Mareo
- Sacos de fluido dentro de los ovarios (quiste ovárico)
- Complicaciones en las mamas (incluye dolor de pecho, tensión mamaria, molestia en las mamas, dolor del pezón e hinchazón del pecho)
- Sofocos
Los siguientes efectos adversos rarosafectan entre 1 y 10 de cada 10.000 pacientes tratados:
- Acné
- Erupción cutánea
Además de los efectos adversos indicados arriba se han comunicado tras la comercialización de Menopury con una frecuencia desconocida, los siguientes efectos adversos:
- Alteraciones en la visión
- Fiebre
- Sentirse mal
- Reacciones alérgicas
- Incremento del peso
- Dolor en el músculo y articulaciones (por ejemplo dolor de espalda, cuello, brazos y piernas)
- Torsión de los ovarios como una complicación del incremento de la actividad de los ovarios debido a la hiperestimulación.
- Prurito
- Urticaria
- Coágulos de sangre como una complicación del incremento de actividad de los ovarios debido a una hiperestimulación.
Comunicación de efectos adversos .
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano https:www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Menopur
Mantener fuera de la vista y del alcance de los niños.
Antes de la reconstitución conservar en nevera (2º C – 8º C). No congelar. Conservar en el envase original para protegerlo de la luz.
Tras la reconstitución, la solución puede conservarse durante un máximo de 28 días a no más de 25º C.
La solución reconstituida no deberá administrarse si contiene partículas o no está transparente.
No utilice Menopur después de la fecha de caducidad que aparece en el envase después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Menopur
El principio activo es menotropina altamente purificada (gonadotropina menopáusica humana, hMG-HP) que corresponde a 600 UI de FSH con actividad hormona folículo estimulante y 600 UI de LH con actividad hormona luteinizante.
Tras la reconstitución, 1 ml de solución reconstituida contiene 600 UI de menotropina altamente purificada.
Los demás componentes del polvo son: lactosa monohidrato, polisorbato 20, fosfato sódico dibásico heptahidratado(como agente tampón y ajuste de pH), y ácido fosfórico (para ajuste pH).
Los componentes del disolvente son: metacresol y agua para inyección.
Aspecto del producto y contenido del envase.
MENOPUR es un polvo y disolvente para solución inyectable.
Menopur es un polvo liofilizado apelmazado blanco a grisáceo que se presenta en un vial de vidrio junto con una jeringa precargada con disolvente, solución transparente incolora, para su reconstitución, 1 aguja para reconstitución y 9 jeringas desechables graduadas en unidades FSH/LH con agujas prefijadas para administración.
Titular de la autorización de comercialización y responsable de la fabricación:
Titular de la autorización de comercialización
Ferring, S.A.U
C/ del Arquitecto Sánchez Arcas nº3, 1º
28040 Madrid
- España.
Responsable de la fabricación:
FERRING GmbH
Wittland 11,
D-24109 Kiel
ALEMANIA
Este prospecto ha sido aprobado enseptiembre 2015
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MENOPUR 600 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, -Principio activo: human menopausal gonadotrophinFabricante: Angelini Pharma Espana S.L.Requiere recetaForma farmacéutica: INYECTABLE, 1200 UIPrincipio activo: human menopausal gonadotrophinFabricante: Ferring S.A.Requiere recetaForma farmacéutica: INYECTABLE, 1.200 UIPrincipio activo: human menopausal gonadotrophinFabricante: Ferring S.A.U.Requiere receta
Médicos online para MENOPUR 600 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MENOPUR 600 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes