

МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИН

Запитайте лікаря про рецепт на МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИН

Інструкція із застосування МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИН
Введення
Опис: інформація для користувача
Мемантин Аресто 10 мг/мл розвідний розчин для перорального прийому ЕФГ
Мемантин, гідрохлорид
Прочитайте уважно весь опис перед тим, як почати прийом цього ліків, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас виникли питання, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей лік призначений тільки вам, і не слід давати його іншим людям, навіть якщо вони мають такі самі симптоми, як і ви, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке Мемантин Аресто і для чого він використовується.
- Що потрібно знати перед тим, як почати прийом Мемантин Аресто.
- Як прийом Мемантин Аресто.
- Можливі побічні ефекти.
- Збереження Мемантин Аресто.
- Зміст упаковки та додаткова інформація.
1. Що таке Мемантин Аресто і для чого він використовується
Як діє Мемантин Аресто
Мемантин Аресто містить активну речовину мемантин гідрохлорид. Це належить до групи ліків, відомих як антидеменційні ліки.
Втрата пам'яті при хворобі Альцгеймера відбувається через порушення сигналів мозку. Мозок містить так звані рецептори N-метил-D-аспартат (NMDA), які беруть участь у передачі важливих нервових сигналів при навчанні та пам'яті. Мемантин належить до групи ліків, званих антагоністами рецепторів NMDA. Мемантин діє на ці рецептори, покращуючи передачу нервових сигналів і пам'яті.
Для чого використовується Мемантин Аресто
Мемантин Аресто використовується для лікування пацієнтів з хворобою Альцгеймера середньої та важкої форми.
2. Що потрібно знати перед тим, як почати прийом Мемантин Аресто
Не прийом Мемантин Аресто
- якщо ви алергічні на мемантин гідрохлорид або на будь-які інші компоненти цього ліків (перелічені в розділі 6).
Попередження та обережність
Проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як почати прийом Мемантин Аресто,
- якщо у вас є анамнез епілептичних нападів (конвульсій).
- якщо ви недавно перенесли інфаркт міокарда (атаку на серце), якщо ви страждаєте на хворобу серця або якщо у вас є неkontrolована гіпертонія (високий тиск).
У цих ситуаціях лікування повинно бути під суворим контролем, і лікар повинен регулярно переоцінювати клінічну користь від Мемантин Аресто.
Якщо у вас є ниркова недостатність (проблеми з нирками), ваш лікар повинен уважно контролювати функцію нирок і, якщо необхідно, коригувати дозу мемантину.
Не слід використовувати мемантин разом з іншими ліками, такими як амантадин (для лікування хвороби Паркінсона), кетамін (загальний анестетик), декстрометорфан (лік для лікування кашлю) та інші антагоністи NMDA.
Діти та підлітки.
Не рекомендується використання Мемантин Аресто у дітей та підлітків молодше 18 років.
Використання Мемантин Арестоз іншими ліками
Проконсультуйтеся з вашим лікарем або фармацевтом, якщо ви приймаєте, приймали недавно або можете приймати інші ліки.
Зокрема, введення Мемантин Аресто може змінити дію інших ліків, тому ваш лікар може потребувати коригування доз:
- амантадин, кетамін, декстрометорфан.
- дантролен, баклофен.
- циметидин, ранітидин, процінамід, хінідин, хінін, нікотин.
- гідрохлортіазид (або будь-яка комбінація з гідрохлортіазидом).
- антіхолінергічні засоби (речовини, які зазвичай використовуються для лікування рухових розладів або кишкових спазмів).
- антіконвульсанти (засоби, які використовуються для профілактики та ліквідації конвульсій).
- барбітурати (речовини, які зазвичай використовуються для індукції сну).
- допамінергічні агоністи (речовини, такі як Л-допа, бромокриптин).
- нейролептики (засоби, які використовуються для лікування психічних захворювань).
- оральні антикоагулянти.
Якщо ви потрапили до лікарні, повідомте вашому лікареві, що ви приймаєте Мемантин Аресто.
Мемантин Аресто з їжею та напоями
Проконсультуйтеся з вашим лікарем, якщо ви недавно змінили або плануєте змінити свою дієту суттєво (наприклад, з нормальної дієти на сувору вегетаріанську дієту) або якщо у вас є кислотоз ниркової трубки (АТР, надмірна кількість кислототворних речовин у крові через ниркову дисфункцію) або важкі інфекції сечовидільної системи (сечовивідний тракт), оскільки ваш лікар може потребувати коригування дози ліків.
Вагітність та лактація
Якщо ви вагітні або годуєте грудьми, вважаєте, що можете бути вагітною або плануєте завагітніти, проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як використовувати цей лік.
Вагітність
Не рекомендується використання мемантину у вагітних жінок.
Годування грудьми
Жінки, які приймають Мемантин Аресто, повинні припинити годування грудьми.
Водіння автомобіля та робота з машинами
Ваш лікар повідомить вам, чи дозволено вам водити автомобіль та працювати з машинами безпечно.
Також Мемантин Аресто може змінити вашу реакцію, тому водіння автомобіля або робота з машинами можуть бути недоцільними.
Мемантин Арестомістить сорбітол (Е 420)
Якщо ваш лікар сказав вам, що у вас є непереносимість певних цукрів, проконсультуйтеся з ним перед тим, як приймати цей лік.
3. Як прийом Мемантин Аресто
Слідуйте точно інструкціям щодо прийому цього ліків, вказаним вашим лікарем. У разі сумнівів проконсультуйтеся знову з вашим лікарем або фармацевтом.
Дозування
0,5 мл розвідного розчину містить 5 мг мемантину гідрохлориду.
Рекомендована доза Мемантин Аресто для дорослих пацієнтів та пацієнтів похилого віку становить 2 мл, що відповідає 20 мг, один раз на добу.
Для зменшення ризику побічних ефектів ця доза досягається поступово згідно з наступним схемою лікування:
Період лікування | Добова доза (мл) |
Тиждень 1 | 0,5 мл один раз на добу (1 х 5 мг) |
Тиждень 2 | 1 мл один раз на добу (1 х 10 мг) |
Тиждень 3 | 1,5 мл один раз на добу (1 х 15 мг) |
Тиждень 4 | 2 мл один раз на добу (1 х 20 мг) |
Звичайна початкова доза становить 0,5 мл (що відповідає 5 мг), приймається один раз на добу протягом першого тижня. Ця доза збільшується до 1 мл (що відповідає 10 мг), приймається один раз на добу, протягом другого тижня, і до 1,5 мл (що відповідає 15 мг), приймається один раз на добу, протягом третього тижня. Починаючи з четвертого тижня, рекомендована доза становить 2 мл (що відповідає 20 мг), приймається один раз на добу.
Дозування для пацієнтів з порушеною нирковою функцією
Якщо у вас є проблеми з нирками, ваш лікар визначить відповідну дозу для вашого стану. У цьому випадку ваш лікар повинен періодично контролювати вашу ниркову функцію.
Застосування
Мемантин Аресто повинен застосовуватися перорально один раз на добу. Для досягнення максимальної користі від вашого ліків ви повинні приймати його щоденно та о同じ час. Розчин можна приймати з їжею або без неї.
Розчин не повинен використовуватися або видалятися безпосередньо з пляшки або шприца. Вимірюйте дозу за допомогою шприца та виливайте її у ложку або у склянку з водою.
Інструкції з використання
- Відкрити пляшку: натисніть на кришку та поверніть у протилежному напрямку до годинникової стрілки.
- Вставте адаптер шприца у горлечок пляшки (Фігура 1). Переконайтеся, що адаптер щільно закріплений.
- Вставте шприц у адаптер (Фігура 1).
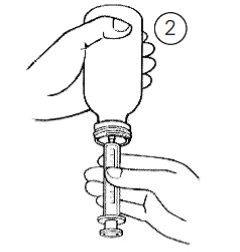
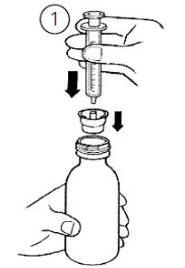 Поверніть пляшку догори дном (Фігура 2).
Поверніть пляшку догори дном (Фігура 2).
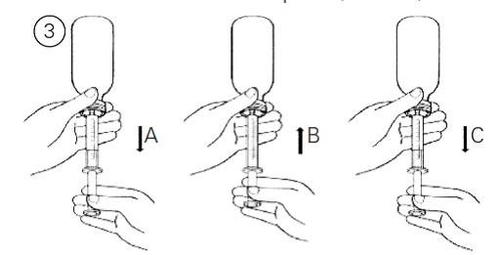
- Наповніть шприц невеликою кількістю розчину, потягнувши повільно поршень (Фігура 3А). Потім потисніть поршень угору, спорожняючи вміст для видалення будь-яких повітряних бульбашок, які могли б утворитися (Фігура 3Б). Після цього пересуньте поршень до позначки у мілілітрах (мл), яка відповідає дозі, призначеній вашим лікарем (Фігура 3В).
- Поставте пляшку вертикально, опираючись на її основу. Видаліть шприц з адаптера.
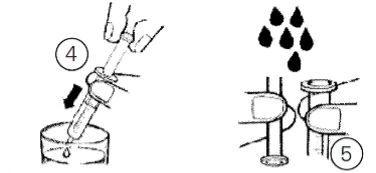
- Виливайте вміст шприца у ложку або у склянку з водою, потиснувши поршень та спорожняючи вміст шприца (Фігура 4).
- Пройміть шприц водою (Фігура 5).
- Закрийте пляшку кришкою.
Тривалість лікування
Продовжуйте прийом Мемантин Аресто, поки це буде вам корисно. Лікар повинен періодично оцінювати ваше лікування.
Якщо ви прийняли надмірну кількість Мемантин Аресто
У разі передозування або випадкового прийому проконсультуйтеся з вашим лікарем або фармацевтом або зверніться до служби токсикологічної інформації, телефон: (91) 5620420, вказавши ліків та кількість, прийняту.
Загалом, прийом надмірної кількості Мемантин Аресто не повинен вам нашкодити. Ви можете відчувати посилення симптомів, описаних у розділі 4 "Можливі побічні ефекти".
Якщо ви забули прийнятиМемантин Аресто
- Якщо ви зрозуміли, що забули прийняти свою дозу Мемантин Аресто, чекайте та прийміть наступну дозу у звичайний час.
Не прийміть подвійну дозу для компенсації забутих доз.
4. Можливі побічні ефекти
Як і всі ліки, цей ліків може викликати побічні ефекти, хоча не всі люди їх відчувають.
Загалом побічні ефекти класифікуються від легких до помірних.
Часті (можуть впливати до1 з10 пацієнтів):
- Головний біль, сонливість, запор, підвищені рівні функції печінки, вертіго, порушення рівноваги, відсутність дихання, підвищений тиск та гіперчутливість до ліків.
Рідкі (можуть впливати до1 з100 пацієнтів):
- Втома, грибкові інфекції, сплутаність свідомості, галюцинації, блювота, порушення ходи, серцева недостатність та утворення тромбів у венозній системі (тромбоз/тромбоемболія).
Дуже рідкі (можуть впливати до 1 з 10 000 пацієнтів):
- Конвульсії.
Частота невідома (частота не може бути оцінена з наявних даних):
- Панкреатит, гепатит (запалення печінки) та психотичні реакції.
Хвороба Альцгеймера пов'язана з депресією, суїцидальними думками та суїцидом. Було повідомлено про виникнення цих подій у пацієнтів, які приймали Мемантин.
Звіт про побічні ефекти
Якщо ви відчуваєте будь-які побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не вказані в цьому описі. Також ви можете повідомити про них безпосередньо через національну систему повідомлення:
Система фармакологічного нагляду за лікарськими засобами для людини Website: www.notificaRAM.es
Інформуючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього ліків.
5. Збереження Мемантин Аресто
Тримайте цей ліків поза досяжністю дітей.
Не використовуйте цей ліків після закінчення терміну придатності, вказаного на коробці та на етикетці пляшки після позначки CAD. Термін придатності - останній день місяця, який вказано.
Не потрібно спеціальних умов зберігання.
Після відкриття вміст пляшки повинен бути використаний протягом 6 місяців.
Ліки не слід викидати у каналізацію або сміття. Відкладайте упаковки та ліки, які вам не потрібні, у пункті SIGREаптеки. У разі сумнівів запитайте у вашого фармацевта, як позбутися упаковок та ліків, які вам не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад Мемантин Аресто
? Активна речовина - мемантин гідрохлорид
? 1 мл розчину містить 10 мг мемантину гідрохлориду, що відповідає 8,31 мг мемантину
- 0,5 мл розчину містить 5 мг мемантину гідрохлориду, що відповідає 4,15 мг мемантину.
- Інші компоненти: сорбат калію, сорбітол рідкий 70% (не кристалізований) (Е420), очищена вода
Вигляд продукту та зміст упаковки
Мемантин Аресто розвідний розчин для перорального прийому - прозорий безбарвний або жовтуватий розчин.
Пляшка з коричневого скла з градуйованим шприцем (марки градуювання 0,5 мл) та адаптером для шприца. Випускається у пляшках по 30 мл, 50 мл або 100 мл розчину.
Клінічна упаковка: 500 мл.
Можливо, що тільки деякі розміри упаковок будуть випускатися.
Уповноважений на отримання дозволу на розміщення ліків на ринку
Aristo Pharma Iberia S.L.
C/ Solana 26
28850 – Torrejón de Ardoz, Madrid
Виробник
Laboratorios Medicamentos Internacionales, S.A. (Medinsa)C/ Solana 26
28850 – Torrejón de Ardoz, Madrid
ARISTO PHARMA GMBH
Wallenroder Strasse 8-10
13435 Berlin, Німеччина
NEURAXPHARM ARZNEIMITTEL GMBH U CO.KG
Elisabethselbert Strasse, 23
Langenfeld – 40764, Німеччина
Цей ліків дозволений у державах-членах Європейського економічного простору під наступними назвами:
Німеччина: Memantin Aristo 10 мг/мл розвідний розчин для перорального прийому
Польща: Memantin NeuroPharma 10 мг/мл розвідний розчин для перорального прийому
Португалія: Memantina Aristo 10 мг/мл розвідний розчин для перорального прийому
Іспанія: Memantina Aristo 10 мг/мл розвідний розчин для перорального прийому ЕФГ
Дата останньої ревізії цього опису: вересень 2017
Детальна інформація про цей ліків доступна на сайті Агентства лікарських засобів та медичних продуктів Іспанії: http://www.aemps.gob.es/
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИНФорма випуску: ТАБЛЕТКА, 10 мгДіючі речовини: memantineВиробник: Merz Pharmaceuticals GmbhПотрібен рецептФорма випуску: ТАБЛЕТКА, 10 мгДіючі речовини: memantineВиробник: Merz Pharmaceuticals GmbhПотрібен рецептФорма випуску: ТАБЛЕТКА, 20 мгДіючі речовини: memantineВиробник: Merz Pharmaceuticals GmbhПотрібен рецепт
Аналоги МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИН в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИН у Польща
Аналог МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИН у Україна
Лікарі онлайн щодо МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИН
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на МЕМАНТИНА АРИСТО 10 мг/мл ОРАЛЬНИЙ РОЗЧИН – за рішенням лікаря та згідно з місцевими правилами.












