Інструкція із застосування ЛЕНАЛІДОМІД Зентіва 15 мг ТВЕРДІ КАПСУЛИ
Вступ
Опис: інформація для користувача
Леналідомід Зентіва 15 мг тверді капсули EFG
леналідомід
Прочитайте уважно весь опис перед тим, як почнете приймати цей препарат, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас виникли питання, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей препарат призначений лише для вас, і його не слід давати іншим людям, навіть якщо вони мають такі самі симптоми, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису:
- Що таке Леналідомід Зентіва і для чого він використовується
- Що вам потрібно знати перед тим, як почати приймати Леналідомід Зентіва
- Як приймати Леналідомід Зентіва
- Можливі побічні ефекти
- Збереження Леналідоміду Зентіва
- Зміст упаковки та додаткова інформація
1. Що таке Леналідомід Зентіва і для чого він використовується
Що такеЛеналідомід Зентіва
Леналідомід Зентіва містить активну речовину "леналідомід". Цей препарат належить до групи препаратів, які впливають на роботу імунної системи.
Для чого використовуєтьсяЛеналідомід Зентіва
Леналідомід використовується у дорослих для:
- Мультіпльної мієломи
- Мієлодиспластичних синдромів (МДС)
- Лімфоми мантійних клітин (ЛМК)
- Фолікулярної лімфоми
Мультіпльна мієлома
Мультіпльна мієлома - це тип раку, який впливає на певний тип білих кров'яних клітин, які називаються плазматичними клітинами. Ці клітини накопичуються в кістковому мозку і розмножуються, виходячи з-під контролю. Це може пошкодити кістки і нирки.
Мультіпльна мієлома зазвичай не має ліків. Однак можна значно зменшити симптоми або зробити їх зникненнями протягом певного періоду часу. Це називається "ремісією".
Мультіпльна мієлома нового діагнозу: у пацієнтів, які пройшли трансплантацію кісткового мозку
Леналідомід використовується як підтримуюче лікування після адекватного відновлення після трансплантації кісткового мозку.
Мультіпльна мієлома нового діагнозу: у пацієнтів, які не можуть бути витерпілими з трансплантацією кісткового мозку
Леналідомід приймається з іншими препаратами:
- хіміотерапевтичним препаратом під назвою "бортезоміб";
- протизапальним препаратом під назвою "дексаметазон";
- хіміотерапевтичним препаратом під назвою "мелфалан" і
- імунодепресивним препаратом під назвою "преднізон".
Ви будете приймати ці препарати на початку лікування, а потім продовжите приймати леналідомід самостійно.
Якщо вам 75 років або більше, або у вас є проблеми з нирками середньої або важкої ступеня, ваш лікар буде ретельно контролювати вас перед початком лікування.
Мультіпльна мієлома: у пацієнтів, які раніше лікувалися
Леналідомід приймається разом з протизапальним препаратом під назвою "дексаметазон".
Леналідомід може зупинити погіршення симптомів мультіпльної мієломи. Також він продемонстрував здатність відстрочувати повторний розвиток мультіпльної мієломи після лікування.
Мієлодиспластичні синдроми (МДС)
МДС - це група багатьох різних захворювань крові і кісткового мозку. Кров'яні клітини стають аномальними і не функціонують правильно. Пацієнти можуть відчувати різноманітні симптоми, серед яких низький рівень червоних кров'яних клітин (анемія), необхідність переливання крові та ризик інфекції.
Леналідомід використовується для лікування пацієнтів з МДС, коли всі наступні пункти застосовні:
- потрібно періодичні переливання крові для лікування низького рівня червоних кров'яних клітин ("анемія, залежна від переливання");
- є аномалія клітин кісткового мозку під назвою "аномація цитогенетична делеція 5q ізольована". Це означає, що ваш організм не виробляє достатню кількість здорових кров'яних клітин;
- інші методи лікування, які ви раніше використовували, не є придатними або не працюють достатньо добре.
Леналідомід може збільшити кількість здорових червоних кров'яних клітин, які виробляє організм, зменшуючи кількість аномальних клітин:
- це може зменшити кількість переливань крові, які необхідні. Можливо, переливання крові не будуть потрібні.
Лімфома мантійних клітин (ЛМК)
ЛМК - це рак імунної системи (лімфатичної тканини). Він впливає на певний тип білих кров'яних клітин, які називаються лімфоцитами Б або клітинами Б. ЛМК - це захворювання, при якому клітини Б ростуть без контролю і накопичуються в лімфатичній тканині, кістковому мозку або крові.
Леналідомід використовується в монотерапії для лікування пацієнтів, які раніше лікувалися іншими препаратами.
Фолікулярна лімфома (ФЛ)
ФЛ - це рак повільного росту, який впливає на лімфоцити Б. Це тип білих кров'яних клітин, які допомагають організму боротися з інфекціями. Коли людина страждає ФЛ, вона може накопичувати надлишок цих лімфоцитів Б в крові, кістковому мозку, лімфатичних вузлах та селезінці.
Леналідомід використовується з іншим препаратом під назвою "ритуксимаб" для лікування пацієнтів, які раніше лікувалися від фолікулярної лімфоми.
Як діє Леналідомід Зентіва
Леналідомід діє, впливаючи на імунну систему організму і безпосередньо на рак.
Він діє різними способами:
- зупиняє розвиток ракових клітин
- зупиняє рост кров'яних судин у пухлині
- стимулює частину імунної системи, щоб вона атакувала ракові клітини.
2. Що вам потрібно знати перед тим, як почати приймати Леналідомід Зентіва
Вам потрібно прочитати опис всіх препаратів, які ви будете приймати разом з леналідомідом, перед тим, як почати лікування леналідомідом.
Не приймають Леналідомід Зентіва:
- якщо ви вагітні, вважаєте, що можете бути вагітні або плануєте завагітніти, оскільки очікується, що леналідомід буде шкідливим для плода(див. розділ 2, "Вагітність, годування грудьми та контрацепція: інформація для жінок і чоловіків").
- якщо ви можете завагітніти, якщо не дотримуєтеся всіх необхідних заходів для避免ання вагітності (див. розділ 2, "Вагітність, годування грудьми та контрацепція: інформація для жінок і чоловіків"). Якщо ви можете завагітніти, ваш лікар зафіксує з кожним рецептом, що були вжиті всі необхідні заходи, і надасть вам цю підтвердження.
- якщо ви алергічні на леналідомід або на будь-який інший компонент цього препарату, перелічений у розділі 6. Якщо ви вважаєте, що можете бути алергічними, проконсультуйтеся з вашим лікарем.
Якщо будь-яка з цих умов застосовна до вас, не приймають леналідомід. У разі сумнівів проконсультуйтеся з вашим лікарем.
Попередження та застереження
Проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою перед тим, як почати приймати леналідомід, якщо:
- ви раніше мали тромби
- у вас є ознаки інфекції, такі як кашель або гарячка
- у вас є або раніше була вірусна інфекція, особливо інфекція вірусом гепатиту Б, вітряної віспи або ВІЛ. У разі сумнівів проконсультуйтеся з вашим лікарем. Лікування леналідомідом може спричинити реактивування вірусу, що призводить до повторного розвитку інфекції. Ваш лікар повинен перевірити, чи ви раніше мали інфекцію вірусом гепатиту Б
- у вас є проблеми з нирками
- ви раніше мали інфаркт міокарда, тромб або якщо ви курите, маєте високий тиск або високий рівень холестерину
- ви раніше мали алергічну реакцію під час прийому талідоміду (інший препарат, який використовується для лікування мультіпльної мієломи), наприклад, висип, свербіж, набряк, головокружіння або проблеми з диханням
- ви раніше мали поєднання будь-яких з наступних симптомів: висип, червоність шкіри, висока гарячка, симптоми грипу, підвищення рівня ферментів печінки, аномалії в крові (еозінофілія), збільшення лімфатичних вузлів (це ознаки важкої алергічної реакції, яка називається синдром ДРЕС)
Якщо будь-яка з цих умов застосовна до вас, повідомте вашому лікарю перед початком лікування.
У будь-який момент, під час або після лікування, повідомте вашому лікарю або медсестрі негайно, якщо ви відчуваєте:
- затьмарення зору, втрату зору або подвійне бачення, труднощі з мовленням, слабкість у руці або нозі, зміни в способі ходьби або проблеми з рівновагою, оніміння, зниження чутливості або втрату чутливості, втрату пам'яті або сплутаність. Все це можуть бути симптомами важкого захворювання головного мозку, яке називається прогресивна мультифокальна лейкоенцефалопатія (ПМЛ). Якщо ви мали будь-який з цих симптомів перед початком лікування леналідомідом, повідомте вашому лікарю, якщо ви помітите будь-які зміни в цих симптомах.
- брак повітря, втома, головокружіння, біль у грудній клітці, прискорене серцебиття або набряк у ногах або ступнях. Все це можуть бути симптомами важкого захворювання, яке називається пульмональна гіпертензія (див. розділ 4).
Аналізи та тести
Перед початком лікування леналідомідом і під час нього вам будуть регулярно проводити аналізи крові, оскільки леналідомід може спричинити зниження рівня клітин крові, які допомагають боротися з інфекціями (білі кров'яні клітини) і тих, які беруть участь у згортанні крові (тромбоцити).
Ваш лікар попросить вас зробити аналіз крові:
- перед початком лікування
- кожного тижня протягом перших 8 тижнів лікування
- пізніше не менше одного разу на місяць.
Вам може бути проведено обстеження для виявлення ознак проблем з серцем і легенями перед і під час лікування леналідомідом.
Для пацієнтів з МДС, які приймають Леналідомід Зентіва
Якщо у вас є МДС, ви можете бути більш схильні до розвитку більш важкого захворювання, яке називається гострий мієлоїдний лейкоз (ГМЛ). Крім того, невідомо, як леналідомід впливає на можливість розвитку ГМЛ. Ваш лікар, тому, може провести аналізи для виявлення ознак, які можуть передбачити розвиток ГМЛ під час лікування леналідомідом.
Для пацієнтів з ЛМК, які приймають Леналідомід Зентіва
Ваш лікар попросить вас зробити аналіз крові:
- перед початком лікування;
- кожного тижня протягом перших 8 тижнів (2 цикли) лікування;
- пізніше кожні 2 тижні у циклах 3 і 4 (див. розділ 3 "Цикл лікування" для отримання більшої інформації);
- пізніше на початку кожного циклу;
- не менше одного разу на місяць.
Для пацієнтів з ФЛ, які приймають Леналідомід Зентіва
Ваш лікар попросить вас зробити аналіз крові:
- перед початком лікування;
- кожного тижня протягом перших 3 тижнів (1 цикл) лікування;
- пізніше кожні 2 тижні у циклах 2-4 (див. розділ 3 "Цикл лікування" для отримання більшої інформації).
- пізніше на початку кожного циклу і
- не менше одного разу на місяць.
Ваш лікар може перевірити, чи у вас є велика кількість пухлинних клітин у організмі, включаючи кістковий мозок. Це може призвести до захворювання, яке називається синдром лізису пухлини (це захворювання, при якому пухлинні клітини розкладаються і виробляють аномальні рівні хімічних речовин у крові, що може призвести до ниркової недостатності).
Ваш лікар може провести обстеження для виявлення змін у вашій шкірі, таких як червоні плями або висип.
Ваш лікар може скоригувати дозу леналідоміду або припинити лікування, залежно від результатів ваших аналізів крові та загального стану. Якщо ви пацієнт з новим діагнозом, ваш лікар також може оцінити ваше лікування залежно від вашого віку та інших захворювань, які у вас є.
Донорство крові
Ви не повинні اهدрувати кров під час лікування та протягом 1 тижня після закінчення лікування.
Діти та підлітки
Не рекомендується використання леналідоміду у дітей та підлітків молодше 18 років.
Люди похилого віку та люди з проблемами нирок
Якщо вам 75 років або більше, або у вас є проблеми з нирками середньої або важкої ступеня, ваш лікар буде ретельно контролювати вас перед початком лікування.
Прийом Леналідоміду Зентіва з іншими препаратами
Повідомте вашому лікарю або медсестрі, якщо ви приймаєте або приймали інші препарати. Це пов'язано з тим, що леналідомід може вплинути на дію інших препаратів. Крім того, деякі препарати можуть вплинути на дію леналідоміду.
Зокрема, повідомте вашому лікарю або медсестрі, якщо ви приймаєте будь-який з наступних препаратів:
- деякі препарати, які використовуються для запобігання вагітності, такі як оральні контрацептиви, оскільки вони можуть перестати діяти
- деякі препарати, які використовуються для лікування проблем з серцем, таких як дигоксин
- деякі препарати, які використовуються для розрідження крові, такі як варфарин
Вагітність, годування грудьми та контрацепція: інформація для жінок і чоловіків
Вагітність
Жінки, які приймають Леналідомід Зентіва
- Не приймають леналідомід, якщо ви вагітні, оскільки очікується, що він буде шкідливим для плода.
- Не повинні завагітніти під час прийому леналідоміду. Тому ви повинні використовувати ефективні методи контрацепції, якщо існує можливість вагітності (див. "Контрацепція" нижче).
- Якщо ви завагітнієте під час лікування леналідомідом, ви повинні припинити лікування і негайно повідомити вашому лікарю.
Чоловіки, які приймають Леналідомід Зентіва
- Якщо ваша партнерка завагітніє під час вашого лікування леналідомідом, ви повинні негайно повідомити вашому лікарю. Рекомендується, щоб ваша партнерка звернулася за медичною консультацією.
- Ви також повинні використовувати ефективні методи контрацепції (див. "Контрацепція" нижче).
Годування грудьми
Не рекомендується годувати грудьми під час прийому леналідоміду, оскільки невідомо, чи леналідомід проникає у грудне молоко.
Контрацепція
Для жінок, які приймають Леналідомід Зентіва
Перед початком лікування запитайте вашого лікаря, чи ви можете завагітніти, навіть якщо ви вважаєте, що це малоймовірно.
Якщо ви можете завагітніти
- вам будуть проводити тести на вагітність під медичним наглядом (перед кожним лікуванням, кожні 4 тижні під час лікування та 4 тижні після закінчення лікування), крім випадків, коли підтверджено закриття фаллопієвих труб, щоб запобігти потраплянню яйцеклітин до матки (лігатура фаллопієвих труб)
І
- ви повинні використовувати ефективні методи контрацепції починаючи з 4 тижнів перед початком лікування, під час лікування та протягом 4 тижнів після закінчення лікування. Ваш лікар порадить вам щодо найбільш підходящих методів контрацепції.
Для чоловіків, які приймають Леналідомід Зентіва
Леналідомід проникає у людський сперму. Якщо ваша партнерка вагітніє або може завагітніти і не використовує жодних ефективних методів контрацепції, ви повинні використовувати презервативи під час лікування та протягом 1 тижня після закінчення лікування, навіть якщо ви пройшли васектомію.
Водіння транспортних засобів та використання машин
Не водьте транспортні засоби та не використовуйте машини, якщо ви відчуваєте головокружіння, втому, сонливість, маєте проблеми з рівновагою або зором після прийому леналідоміду.
Леналідомід Зентіва містить лактозу
Цей препарат містить лактозу. Якщо ваш лікар сказав вам, що ви маєте непереносимість деяких цукрів, проконсультуйтеся з ним перед прийомом цього препарату.
Цей препарат містить менше 1 ммоль натрію (23 мг) на капсулу; це означає, що він практично не містить натрію.
3. Як приймати/вживляти Леналідомід Зентіва
Леналідомід повинен бути призначений кваліфікованим медичним працівником, який має досвід лікування множинної мієломи, СМД, ЛЦМ або ЛФ.
- Коли леналідомід використовується для лікування множинної мієломи у пацієнтів, які не можуть бути піддані трансплантації кісткового мозку або пройшли інші методи лікування раніше, його приймають разом з іншими лікарськими засобами (див. розділ 1).
- Коли леналідомід використовується для лікування множинної мієломи у пацієнтів, які пройшли трансплантацію кісткового мозку або для лікування пацієнтів із СМД або ЛЦМ, його приймають самостійно.
- Коли леналідомід використовується для лікування фолікулярного лімфому, його приймають разом з іншим лікарським засобом під назвою "ритуксимаб".
Слідувати точно інструкціям щодо прийому леналідоміда, які вказав ваш лікар. У разі сумнівів зверніться до вашого лікаря або фармацевта.
Якщо ви приймаєте леналідомід разом з іншими лікарськими засобами, ви повинні ознайомитися з інструкцією цих інших лікарських засобів, щоб отримати додаткову інформацію про їх використання та побічні ефекти.
Цикл лікування
Леналідомід приймають певні дні протягом періоду 3 тижнів (21 день).
- «Цикл лікування» складається з 21 дня.
- Залежно від дня циклу, ви прийматимете один або кілька лікарських засобів. Однак деякими днями ви не прийматимете жодного лікарського засобу.
- Після закінчення кожного циклу з 21 дня ви повинні розпочати новий «цикл» протягом наступних 21 днів
Або
Леналідомід приймають певні дні протягом періоду 4 тижнів (28 днів).
- «Цикл лікування» складається з 28 днів.
- Залежно від дня циклу, ви прийматимете один або кілька лікарських засобів. Однак деякими днями ви не прийматимете жодного лікарського засобу.
- Після закінчення кожного циклу з 28 днів ви повинні розпочати новий «цикл» протягом наступних 28 днів.
Як приймати Леналідомід Зентіва
Перед початком лікування ваш лікар призначить:
- яку кількість леналідоміда ви повинні приймати
- яку кількість інших лікарських засобів ви повинні приймати разом з леналідомідом, якщо це необхідно
- які дні циклу лікування ви повинні приймати кожний лікарський засіб.
Як і коли приймати Леналідомід Зентіва
- проглотіть капсули цілі, бажано з водою.
- не розбивайте, не відкривайте та не жуйте капсули. У разі потрапляння порошку розбитої капсули леналідоміда на шкіру, миттєво та ретельно промийте шкіру водою та мильним розчином.
- Медичні працівники, доглядачі та члени сім'ї повинні використовувати одноразові рукавички при обробці блистеру або капсули. Після цього рукавички повинні бути видалені з обережністю, щоб уникнути контакту шкіри, поміщені у пластиковий пакет та викинуті згідно з місцевими вимогами. Руки повинні бути ретельно промиті водою та мильним розчином. Жінки, які перебувають у стані вагітності або підозрюють, що можуть бути вагітними, не повинні обробляти блистер чи капсулу.
- капсули можна приймати з або без їжі.
- ви повинні приймати леналідомід приблизно в один і той же час у призначені дні.
Прийом цього лікарського засобу
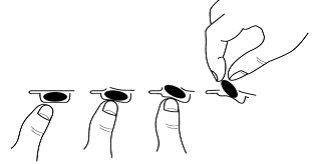
Для видалення капсули з блистеру:
- натисніть тільки на один кінець капсули, щоб вона вийшла через плівку.
- не натискуйте на центр капсули, оскільки це може призвести до її розбиття.
Тривалість лікування Леналідомідом Зентіва
Леналідомід приймають у циклах лікування, кожен цикл триває 21 або 28 днів (див. «Цикл лікування» вище). Ви повинні продовжувати цикли лікування до тих пір, поки ваш лікар не повідомить вам про припинення лікування.
Якщо ви прийняли більше Леналідоміда Зентіва, ніж потрібно
Якщо ви прийняли більше леналідоміда, ніж вам призначили, негайно повідомте про це вашому лікареві.
У разі передозування або випадкового прийому лікарського засобу зверніться негайно до вашого лікаря, фармацевта або служби токсикологічної інформації, телефон: 91 562 04 20, вказавши назву лікарського засобу та кількість, прийняту.
Якщо ви забули прийняти Леналідомід Зентіва
Якщо ви забули прийняти леналідомід у звичайний час і
- мініть менше 12 годин: прийміть капсулу негайно.
- мініть більше 12 годин: не прийміть капсулу. Прийміть наступну капсулу на наступний день у звичайний час.
Якщо у вас є будь-які інші питання щодо використання цього лікарського засобу, зверніться до вашого лікаря або фармацевта.
4. Можливі побічні ефекти
Як і всі лікарські засоби, леналідомід може викликати побічні ефекти, хоча не всі люди їх відчувають.
Перестаньте приймати леналідомід і зверніться до лікаря негайно, якщо ви помітите будь-які з наступних серйозних побічних ефектів; можливо, вам буде потрібне термінове медичне лікування:
- Кропив'янка, висипання на шкірі, набряк очей, рота або обличчя, труднощі з диханням або свербіння, які можуть бути симптомами серйозних алергічних реакцій, таких як ангіоедем та анафілактична реакція.
- Серйозна алергічна реакція, яка може початися як висипання на одному місці, але потім поширюється з великою втратою шкіри по всьому тілу (синдром Стівенса-Джонсона та/або токсична епідермальна некроліз).
- Висипання на шкірі, підвищена температура тіла, підвищення рівня ферментів печінки, аномалії крові (еозінофілія), збільшення лімфатичних вузлів та ураження інших органів тіла (фармакологічна реакція з еозінофілією та системними симптомами, також відома як DRESS або синдром лікарської гіперчутливості). Див. також розділ 2.
Негайно повідомте про це вашому лікареві, якщо ви відчуваєте будь-які з наступних побічних ефектів:
- Гарячка, озноб, біль у горлі, кашель, виразки у роті або будь-які інші симптоми інфекції, включаючи інфекцію кровотоку (сепсис)
- Кровотеча (кровотеча) або гематома (синяк) не через травму
- Біль у грудній клітці (торакальний) або в ногах
- Труднощі з диханням
- Біль у кістках, слабкість м'язів, сплутаність або втома, які можуть бути симптомами високого рівня кальцію у крові.
Леналідомід може зменшити кількість білих кров'яних тілець (клітин крові, які борються з інфекціями), червоних кров'яних тілець (клітин крові, які переносять кисень) та також плакет (клітин, які допомагають згортатися крові). Леналідомід також може викликати кров'яні згортання у венах (тромбоз).
Інші побічні ефекти
Важливо зазначити, що обмежена кількість пацієнтів може розвивати інші види раку, і цей ризик може збільшуватися при лікуванні леналідомідом. Тому ваш лікар повинен ретельно оцінити користь і ризик при призначенні леналідоміда.
Побічні ефекти дуже часто(можуть впливати на більше 1 з 10 осіб):
- Зменшення кількості червоних кров'яних тілець, що може призвести до анемії, яка викликає втому та слабкість.
- Висипання на шкірі, свербіння
- М'язові спазми, слабкість м'язів, біль у м'язах, незручність у м'язах, біль у кістках, біль у суглобах, біль у спині, біль у кінцівках.
- Загальне набрякання, яке включає набряк рук і ніг.
- Слабкість, втома.
- Гарячка та псевдогрипозні симптоми, які включають гарячку, біль у м'язах, головний біль, біль у вухах, кашель та озноб.
- Оніміння, поколювання або відчуття печіння на шкірі, болі у руках або ногах, головокружіння, тремор,
- Зменшення апетиту, зміни смаку.
- Збільшення болю, розміру пухлини або червоного кольору навколо пухлини.
- Втрата ваги.
- Запор, діарея, нудота, блювота, біль у животі, кислотність шлунку.
- Низький рівень калію або кальцію та/або натрію у крові.
- Погіршення функції щитоподібної залози.
- Біль у ногах (що може бути симптомом тромбозу), біль у грудній клітці або труднощі з диханням (що може бути симптомом легеневого згортання, відомого як легенева емболія).
- Інфекції будь-якого типу, включаючи інфекцію синусів, які оточують ніс (синусит), інфекцію легень та верхніх дихальних шляхів.
- Труднощі з диханням.
- Замазаний зір.
- Замутнення очей (катаракта).
- Ниркові проблеми, які включають нирки, які не функціонують правильно або не можуть підтримувати нормальну діяльність.
- Аномальні результати лабораторних тестів на функцію печінки.
- Високі значення у результатах лабораторних тестів на функцію печінки.
- Зміни у білку крові, який може викликати набряк артерій (васкуліт).
- Збільшення рівня цукру у крові (цукровий діабет).
- Зменшення рівня цукру у крові.
- Головний біль.
- Носова кровотеча.
- Суха шкіра.
- Депресія, зміни настрою, труднощі з сном.
- Кашель.
- Зниження артеріального тиску.
- Розмита відчуття нездоров'я у тілі, відчуття нездоров'я.
- Болючий та сухий рот.
- Зневоднення.
Побічні ефекти часто(можуть впливати до 1 з 10 осіб):
- Руйнування червоних кров'яних тілець (гемолітична анемія).
- Певні види шкірних пухлин.
- Кровотеча з ясен, шлунку або кишечника.
- Збільшення артеріального тиску, повільне, швидке або нерегулярне серцебиття.
- Збільшення кількості речовини, яка виділяється після нормального або аномального руйнування червоних кров'яних тілець.
- Збільшення рівня білка, який вказує на запалення в організмі.
- Затемнення кольору шкіри; зміна кольору шкіри внаслідок внутрішнього кровотечі, зазвичай викликаного синяками; запалення шкіри, викликане накопиченням крові; синяк.
- Збільшення рівня сечовини у крові.
- Висипання на шкірі, червоність шкіри, тріщини на шкірі, лущення або відшарування шкіри, кропив'янка.
- Збільшення потовиділення, нічне потовиділення.
- Труднощі з ковтанням, біль у горлі, труднощі з підтриманням якості голосу або зміни голосу.
- Носова кровотеча.
- Значне збільшення або зменшення кількості сечі порівняно з нормою або нездатність контролювати сечовипускання.
- Кров у сечі.
- Труднощі з диханням, особливо при лежанні (що може бути симптомом серцевої недостатності).
- Труднощі з досягненням ерекції.
- Інсульт, непритомність, вертіго (розлад внутрішнього вуха, який викликає відчуття, що все обертається), тимчасова втрата свідомості.
- Біль у грудній клітці, який поширюється на руки, шию, щелепу, спину або живіт, відчуття поту та нестачі повітря, нудота або блювота, які можуть бути симптомами інфаркту міокарда.
- Слабкість м'язів, відсутність енергії.
- Біль у шиї, біль у грудній клітці.
- Озноб.
- Набряк суглобів
- Зменшення потоку жовчі з печінки або блокування.
- Низький рівень фосфату або магнію у крові.
- Труднощі з мовленням.
- Пошкодження печінки.
- Розлад рівноваги, труднощі з рухами.
- Глухота, звон у вухах (тінітус).
- Біль у нервах, незвичайне та неприємне відчуття, особливо при дотику.
- Перевищення рівня заліза в організмі.
- Спрага.
- Замішання.
- Біль у зубах.
- Падіння, яке може викликати травми.
Побічні ефекти рідко(можуть впливати до 1 з 100 осіб):
- Кровотеча всередині черепа.
- Проблеми з циркуляцією крові.
- Втрата зору.
- Втрата статевого потягу (лібідо).
- Видалення великої кількості сечі з болем у кістках та слабкістю, які можуть бути симптомами ниркової дисфункції (синдром Фанконі).
- Жовтіння шкіри, слизових оболонок або очей (жовтяниця), блідий колір фекалій, темний колір сечі, свербіння шкіри, висипання, біль або набряк живота; ці можуть бути симптомами пошкодження печінки (печінкова недостатність).
- Біль у животі, набряк живота або діарея, які можуть бути симптомами запалення товстої кишки (коліт або тифліт).
- Пошкодження ниркових клітин (називається рубкова некроз нирок)
- Зміни кольору шкіри, чутливість до сонячного світла.
- Синдром лізису пухлини – можуть виникнути метаболічні ускладнення під час лікування раку та іноді навіть без лікування. Ці ускладнення виникають внаслідок продуктів розпаду пухлинних клітин, які гинуть, і можуть включати: зміни у біохімії крові, високі рівні калію, фосфору, сечовини та низькі рівні кальцію, які, таким чином, викликають зміни у функції нирок та серцевому ритмі, судоми та іноді смерть.
- Збільшення артеріального тиску всередині кров'яних судин, які живлять легені (легенева гіпертензія).
Частота невідома(не може бути оцінена на основі доступних даних):
- Раптовий біль або легкий біль у верхній частині живота та/або спині, який триває кілька днів, можливо, супроводжується нудотою, блювотою, гарячкою та швидким пульсом. Ці симптоми можуть бути викликані запаленням підшлункової залози.
- Свист або звон у вухах при диханні, труднощі з диханням або сухий кашель, які можуть бути симптомами, викликаними запаленням легеневого тканини.
- Було зафіксовано рідкі випадки руйнування м'язів (біль, слабкість або набряк м'язів), яке може призвести до ниркових проблем (рабдоміоліз), деякі з них при прийомі леналідоміда з статинами (тип лікарського засобу для зниження рівня холестерину).
- Хвороба, яка впливає на шкіру, викликана запаленням малих кров'яних судин, супроводжується болем у суглобах та гарячкою (васкуліт лейкоцитокластичний).
- Перфорування стінки шлунку або кишечника. Це може призвести до дуже серйозної інфекції. Повідомте про це вашому лікареві, якщо у вас є сильний біль у животі, гарячка, нудота, блювота, кров у фекаліях або зміни у кишкових звичаях.
- Вірусні інфекції, які включають герпес зостер (також відомий як «кулеบรіла», вірусна хвороба, яка викликає болючу висипання з пухирями) та повторне виникнення інфекції вірусом гепатиту Б (що може викликати жовтіння шкіри та очей, темний колір сечі, біль у правому боці живота, гарячку або нудоту чи відчуття нездоров'я).
- Відхилення трансплантату твердих органів (наприклад, нирок, серця).
Повідомлення про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, зверніться до вашого лікаря, фармацевта або медсестри, навіть якщо це можливі побічні ефекти, які не перелічені в цьому листку. Ви також можете повідомити про них безпосередньо через Систему фармакологічного нагляду за лікарськими засобами для людини: https://www.notificaram.es. Надсилаючи повідомлення про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Зберігання Леналідоміда Зентіва
Тримайте цей лікарський засіб поза досяжністю дітей.
Не використовувати цей лікарський засіб після закінчення терміну придатності, вказаного на блистеру та коробці після «CAD»/«EXP». Термін придатності – останній день місяця, який вказано.
Цьому лікарському засобу не потрібні спеціальні умови зберігання.
Не використовувати цей лікарський засіб, якщо ви помітите видимі ознаки псування або ознаки неправильної обробки.
Лікарські засоби не повинні викидатися у водопровідні труби або сміття. У разі сумнівів зверніться до вашого фармацевта, щоб дізнатися, як позбутися упаковок та лікарських засобів, які вам не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад Леналідоміда Зентіва 15 мг твердих капсул EFG
- Активний інгредієнт – леналідомід. Кожна капсула містить 15 мг леналідоміда.
- Інші компоненти:
- Зміст капсул: лактоза (див. розділ 2), мікрокристалічна целюлоза, содій карбоксиметилцелюлоза та стеарат магнію.
- Покриття капсули: чорний оксид заліза (E172), червоний оксид заліза (E172), жовтий оксид заліза (E172), діоксид титану (E171) та желатина
- Друк фарби: лак (шеллак), пропіленгліколь (E1520), гідроксид калію та чорний оксид заліза (E172)
Вигляд продукту та вміст упаковки
Твердих капсул 15 мг мають кришку непрозору м'ятного кольору та тіло непрозоре сірого кольору, з розміром покриття капсули № 2, 18-19 мм, надруковані чорною фарбою з «LP» на кришці та «640» на тілі.
Капсули поставляються в картонних коробках з блистерами з поліхлорвінілу (ПВХ) / поліхлортрифторетилену (ПКТФЕ) / алюмінію по 7 капсул кожний.
21 капсула в блистерах з ПВХ/ПКТФЕ/Алюмінію.
21х1 капсула в блистерах унідозових перфорованих ПВХ/ПКТФЕ/Алюмінію.
7 капсул в блистерах з ПВХ/ПКТФЕ/Алюмінію
Можливо, що тільки деякі розміри упаковок будуть випущені на ринок.
Уповноважений на отримання дозволу на продаж та відповідальний за виробництво
Уповноважений на отримання дозволу на продаж
Zentiva k.s.,
U kabelovny 130,
Dolní Mecholupy,
102 37 Прага 10
Чехія
Відповідальний за виробництво
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park,
Paola PLA 3000,
Мальта
або
Aristo Pharma GmbH
Wallenroder Straße 8-10
13435 Берлін
Німеччина
Ви можете звернутися за додатковою інформацією про цей лікарський засіб до місцевого представника уповноваженого на отримання дозволу на продаж: Zentiva Spain S.L.U.
Avenida de Europa, 19, Edificio 3, Planta 1.
28224 Pozuelo de Alarcón, Мадрид
Іспанія
Цей лікарський засіб дозволений до продажу в державах-членах ЄЕЗ під наступними назвами:
Ісландія Lenalidomid Aristo 15 мг твердих капсул
Австрія Lenalidomid Aristo 15 мг твердих капсул
Хорватія Lenalidomid Aristo 15 мг твердих капсул
Данія Lenalidomid Aristo
Німеччина Lenalidomid Aristo 15 мг твердих капсул
Італія Lenalidomid Aristo
Норвегія Lenalidomid Aristo 15 мг твердих капсул
Іспанія Леналідомід Зентіва 15 мг твердих капсул EFG
Швеція Lenalidomid Aristo 15 мг твердих капсул
Дата останнього перегляду цього листка:Липень 2022
Детальна інформація про цей лікарський засіб доступна на сайті Іспанського агентства з лікарських засобів та медичних продуктів (AEMPS) http://www.aemps.gob.es/
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ЛЕНАЛІДОМІД Зентіва 15 мг ТВЕРДІ КАПСУЛИФорма випуску: КАПСУЛА, 10 мгДіючі речовини: lenalidomideВиробник: Accord Healthcare S.L.U.Потрібен рецептФорма випуску: КАПСУЛА, 15 мгДіючі речовини: lenalidomideВиробник: Accord Healthcare S.L.U.Потрібен рецептФорма випуску: КАПСУЛА, 2,5 мгДіючі речовини: lenalidomideВиробник: Accord Healthcare S.L.U.Потрібен рецепт
Аналоги ЛЕНАЛІДОМІД Зентіва 15 мг ТВЕРДІ КАПСУЛИ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог ЛЕНАЛІДОМІД Зентіва 15 мг ТВЕРДІ КАПСУЛИ у Польща
Аналог ЛЕНАЛІДОМІД Зентіва 15 мг ТВЕРДІ КАПСУЛИ у Україна
Лікарі онлайн щодо ЛЕНАЛІДОМІД Зентіва 15 мг ТВЕРДІ КАПСУЛИ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ЛЕНАЛІДОМІД Зентіва 15 мг ТВЕРДІ КАПСУЛИ – за рішенням лікаря та згідно з місцевими правилами.