
FEMMYN 0,03 MG ÓVULOS

Cómo usar FEMMYN 0,03 MG ÓVULOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para la usuaria
Femmyn 0,03 mg óvulos
Estriol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Femmyn 0,03 mg óvulos y para qué se utiliza
- Qué necesita saber antes de empezar a usar Femmyn 0,03 mg óvulos
- Cómo usar Femmyn 0,03 mg óvulos
- Posibles efectos adversos
- Conservación de Femmyn 0,03 mg óvulos
- Contenido del envase e información adicional
1. Qué es Femmyn 0,03 mg óvulos y para qué se utiliza
Femmyn pertenece a un grupo de medicamentos denominados Terapia Hormonal Sustitutiva (THS) vaginal.
Se utiliza para aliviar los síntomas menopáusicos en la vagina como la sequedad o la irritación. En términos médicos esto se conoce como “atrofia vaginal”. Está causada por un descenso en los niveles de estrógenos en su cuerpo. Esto sucede de forma natural después de la menopausia.
Femmyn actúa reemplazando los estrógenos que se producen de forma normal en los ovarios de las mujeres. Se inserta en su vagina, de forma que la hormona se libera donde se necesita. Eso puede aliviar las molestias en la vagina.
Femmyn no es un anticonceptivo, ni reestablece la fertilidad.
2. Qué necesita saber antes de empezar a usar Femmyn 0,03 mg óvulos
Historia médica y revisiones
El uso de THS conlleva riesgos que se deben tener en cuenta a la hora de decidir si empezar a tomarlos o continuar el tratamiento.
La experiencia en el tratamiento de mujeres con menopausia prematura (debida a un fallo del funcionamiento de los ovarios o provocada quirúrgicamente) es limitada. Si padece menopausia prematura, los riesgos del uso de THS pueden ser diferentes. Consulte a su médico.
Antes de iniciar (o reiniciar) el tratamiento con este medicamento, su médico le formulará una serie de preguntas sobre su historia médica personal y familiar. Su médico puede considerar adecuado hacerle una revisión física. Esta exploración física podría comprender una exploración de las mamas y/o una exploración interna, si fuera necesario.
Una vez haya iniciado el tratamiento con Femmyn, deberá acudir a su médico para revisiones periódicas (al menos una vez al año). En estas revisiones, puede hablar con su médico sobre los beneficios y los riesgos de seguir el tratamiento con Femmyn.
Sométase a controles regulares de mama, según le recomiende su médico
No use Femmyn
Si se encuentra dentro de alguna de las siguientes situaciones. Si no está segura, consulte con su médicoantes de utilizar este medicamento:
- Si padece o ha padecido cáncer de mama, o si sospecha que pueda tenerlo.
- Si padece cáncer sensible a los estrógenos, como el cáncer del revestimiento interno del útero (endometrio), o si sospecha que lo padece.
- Si presenta hemorragias vaginales anormales.
- Si padece un excesivo engrosamiento del revestimiento interno del útero(hiperplasia endometrial) para el que no está recibiendo tratamiento.
- Si tiene o ha tenido alguna vez un coágulo de sangre en una vena(trombosis) como por ejemplo en las piernas (trombosis venosa profunda) o en los pulmones (embolia pulmonar).
- Si tiene un trastorno que afecte a la coagulación de la sangre(como falta de proteína C, de proteína S o de antitrombina).
- Si padece o ha padecido recientemente una enfermedad causada por coágulos de sangre en las arterias, como un infarto de miocardio, una apoplejíao una angina de pecho.
- Si padece o ha padecido algún trastorno grave del hígadoy las pruebas de función hepática no han vuelto a la normalidad.
- Si padece un problema en la sangre poco frecuente llamado “porfiria” que se transmite entre los miembros de una familia (hereditario).
- Si es alérgicaal estriolo a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Si alguna de las situaciones mencionadas aparece por primera vez mientras está usando este medicamento, deje de tomarlo en el acto y consulte inmediatamente a su médico.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Femmyn. Informe a su médico si tiene o ha tenido alguna de las siguientes enfermedades antes de empezar el tratamiento, ya que pueden aparecer de nuevo o empeorar durante el tratamiento con Femmyn. Si este es el caso, debería acudir más a menudo a su médico para hacerse revisiones:
- Fibromas en el útero.
- Crecimiento del revestimiento interno del útero por fuera del mismo (endometriosis) o antecedentes de un crecimiento excesivo del revestimiento interno del útero (hiperplasia endometrial).
- Aumento del riesgo de aparición de coágulos en la sangre (ver “Coágulos de sangre en una vena (trombosis)”).
- Aumento del riesgo de padecer cáncer sensible a los estrógenos (como tener una madre, hermana o abuela que haya padecido cáncer de mama).
- Tensión arterial elevada.
- Trastornos del hígado, como un tumor benigno en el hígado.
- Diabetes.
- Cálculos biliares.
- Migraña o dolor de cabeza (intenso).
- Enfermedad del sistema inmunitario que afecta a muchas partes del cuerpo (lupus eritematoso sistémico, LES).
- Epilepsia.
- Asma.
- Enfermedad que afecte al tímpano y al oído (otosclerosis).
- Niveles muy altos de grasa en la sangre (triglicéridos).
- Retención de líquidos por problemas cardiacos o renales.
- Angioedema hereditario y adquirido.
Antes de empezar el tratamiento con este medicamento se deben tratar las infecciones vaginales con los medicamentos apropiados.
Deje de usar Femmyny contacte inmediatamente con su médico
Si experimenta cualquiera de los siguientes síntomas cuando esté tomando THS:
- Si aparece cualquiera de las situaciones indicadas en la sección “No use Femmyn”.
- Coloración amarillenta de la piel o de la zona blanca de los ojos (ictericia). Estos pueden ser signos de enfermedad del hígado.
- Gran aumento de la tensión arterial (los síntomas pueden ser dolor de cabeza, cansancio, mareos).
- Si tiene por primera vez una migraña o dolores de cabeza intensos.
- Embarazo.
- Si observa síntomas de trombosis como
- dolor e hinchazón en las piernas
- dolor repentino en el pecho
- o dificultad para respirar
Para más información, ver “Coágulos de sangre en una vena (trombosis)”.
Si aparece cualquiera de las situaciones mencionadas, su médico puede tener que pedirle que interrumpa el tratamiento e indicarle otro alternativo.
Observación
Femmyn no es un anticonceptivo. Si han transcurrido menos de 12 meses desde su última menstruación o tienen menos de 50 años, puede que necesite usar un método anticonceptivo adicional para evitar un embarazo. Pregunte a su médico.
THS y cáncer
Crecimiento excesivo del revestimiento interno del útero (hiperplasia endometrial) y cáncer del revestimiento interno del útero (cáncer de endometrio)
La toma de comprimidos de THS de estrógenos solos durante un largo período de tiempo puede incrementar el riesgo de desarrollar cáncer del revestimiento interno del útero (el endometrio).
No está claro si hay un riesgo similar si Femmyn se usa para tratamientos repetidos o a largo plazo (más de un año). No obstante, Femmyn ha mostrado tener una absorción muy baja en la sangre, por lo cual la adición de progestágeno no es necesaria.
Puede aparecer sangrado o manchado, normalmente no es motivo de preocupación, pero consulte a su médico. Puede ser un síntoma que su endometrio se está engrosando.
Los siguientes riesgos son aplicables a medicamentos de terapia hormonal sustitutiva (THS) que se pasan al torrente sanguíneo. Femmyn se usa para el tratamiento local en la vagina y la absorción a la sangre es muy baja. Es menos probable que las situaciones descritas a continuación empeoren o reaparezcan durante el tratamiento con Femmyn, pero debe visitar a su médico si le afectan.
Cáncer de mama
Los datos sugieren que usar Femmyn no aumenta el riesgo de cáncer de mama en mujeres que no hayan tenido cáncer de mama en el pasado. No se sabe si Femmyn puede usarse de forma segura en mujeres que hayan tenido cáncer de mama en el pasado.
Revise regularmente sus mamas. Visite a su médico si detecta cualquier cambio como:
- Hoyuelos en la piel.
- Cambios en el pezón.
- Cualquier bulto que pueda ver o sentir.
Adicionalmente, puede realizarse mamografías cuando se le sugiera.
Cáncer de ovario
El cáncer de ovario es raro – mucho más raro que el cáncer de mama. El uso de THS de estrógenos solos se ha asociado con un riesgo ligeramente superior de padecer cáncer de ovario.
El riesgo de cáncer de ovario varía con la edad. Por ejemplo, en mujeres de 50 a 54 años de edad que no están en tratamiento con THS, alrededor de 2 mujeres de cada 2.000 serán diagnosticadas de cáncer de ovario en un período de 5 años. En mujeres en tratamiento con THS durante 5 años, se han observado alrededor de 3 casos por cada 2.000 mujeres (es decir, alrededor de un caso adicional).
Efecto de la THS en el corazón y circulación
Coágulos de sangre en una vena (trombosis)
El riesgo de tener coágulos de sangre en las venases aproximadamente entre 1,3 y 3 veces superior en usuarias de THS que en las que no lo son, especialmente durante el primer año de su uso.
Los coágulos de sangre pueden ser graves, y si uno se desplaza hasta los pulmones, puede causar dolor en el pecho, dificultad al respirar, desfallecimiento (desmayos) e incluso la muerte.
Se es más propenso a tener un coágulo de sangre en las venas a medida que se envejece y si cumple alguna de las siguientes circunstancias. Informe a su médico si padece alguna de estas situaciones:
- No puede caminar durante un tiempo largo debido a una intervención quirúrgica mayor, por una lesión (daño) o por una enfermedad (ver también la sección 3, Si tiene que someterse a una intervención de cirugía (operación).
- Si tiene un importante sobrepeso (índice de masa corporal > 30 Kg/m2.
- Si tiene algún problema de coagulación de la sangre que requiere un tratamiento a largo plazo con un medicamento usado para prevenir coágulos de sangre.
- Si alguno de sus parientes cercanos ha tenido alguna vez un coágulo sanguíneo en la pierna, pulmón u otro órgano.
- Si padece lupus eritematoso sistémico (LES).
- Si padece cáncer.
Para más información sobre los síntomas de formación de un coágulo en la sangre, ver “Deje de usar Femmyn y contacte inmediatamente con su médico”.
Datos comparativos
Por término medio, en un periodo de 5 años, entre mujeres que están en el tramo de edad de los 50 años y que no están tomando THS, se espera que de 4 a 7 de cada 1.000 desarrollen un coágulo de sangre en las venas.
Entre mujeres que están en el tramo de edad de los 50 años y que han estado tomando THS de estrógeno solo durante más de 5 años, habrá de 5 a 8 casos de cada 1.000 usuarias (es decir, 1 caso adicional).
Enfermedad cardíaca (infarto de miocardio)
Para mujeres que tomen terapia de estrógenos solos no hay un riesgo incrementado de desarrollar una enfermedad cardíaca.
Ictus
El riesgo de padecer un ictus es de 1,5 veces superior en las usuarias de THS que en las no usuarias. El número de casos adicionales de ictus debido al uso de THS aumentará con la edad.
Datos comparativos
Entre las mujeres que están en el tramo de edad de los 50 años y que no están tomando THS, por término medio, se esperaría que 8 de cada 1.000 sufrieran un ictus en un periodo de 5 años. Entre las mujeres que están en el tramo de edad de los 50 años y que usan THS, habrá 11 casos de cada 1.000 usuarias en un periodo de 5 años (es decir, 3 casos adicionales).
Otras advertencias
La THS no impedirá la pérdida de memoria. Existen algunos indicios de un mayor riesgo de pérdida de memoria en mujeres que empiezan a usar THS después de los 65 años. Pida consejo a su médico.
Uso de Femmyn 0,03 mg óvulos con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento, incluyendo medicamentos sin receta, medicamentos a base de plantas u otros productos naturales.
Este medicamento se usa para el tratamiento local en la vagina y no se esperan interacciones con otros medicamentos tras la administración de este medicamento.
Este medicamento puede afectar otros tratamientos aplicados en la vagina pero es poco probable que afecte a otros medicamentos.
El uso simultáneo de este medicamento con preservativos de látex puede reducir la tensión de éstos y afectar la seguridad proporcionada por los preservativos.
Embarazo y lactancia
Femmyn está indicado para mujeres postmenopáusicas. Si se queda embarazada, deje de tomar este medicamento y contacte con su médico.
Conducción y uso de máquinas
La influencia de este medicamento sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
Femmyn contiene butilhidroxitolueno
Este medicamento puede producir reacciones locales en la piel (como dermatitis de contacto) o irritación de los ojos y membranas mucosas porque contiene butilhidroxitolueno.
3. Cómo usar Femmyn 0,03 mg óvulos
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es:
La dosis recomendada es de un óvulo al día (correspondiente a 0,03 mg de estriol diario), vía vaginal, durante las 3 primeras semanas de tratamiento. Después se recomienda una dosis de mantenimiento de un óvulo dos veces por semana.
Su médico procurará recetarle la dosis más baja para tratar sus síntomas y durante el menor tiempo necesario. Consulte a su médico si cree que esta dosis es demasiado fuerte o que no es suficientemente fuerte para usted.
Método y duración de la administración
El óvulo se debe introducir profundamente en la vagina, preferiblemente por la noche antes de acostarse.
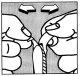
Para administrar el óvulo, separe las dos láminas hacia los lados hasta que sea posible extraer el óvulo fácilmente.
Si tiene que someterse a una intervención de cirugía (operación)
Si va a someterse a una intervención de cirugía, informe al cirujano que está usando Femmyn. Podría ser necesario que dejara de usar Femmyn durante aproximadamente un periodo de 4 a 6 semanas antes de la operación para reducir el riesgo de formación de un coágulo en la sangre (ver sección 2, "Coágulos de sangre en una vena"). Pregunte a su médico cuándo puede empezar a usar de nuevo Femmyn.
Si usa más Femmyn del que debe
No debe preocuparse si en alguna ocasión se administra más óvulos de los necesarios, no obstante consulte a su médico. Los síntomas que pueden aparecer son náuseas y vómitos, y también puede producirse sangrado vaginal después de algunos días.
En caso de sobredosis o ingestión accidental, consulte con su médico o farmacéutico o llame al Servicio de Información Toxicológica. Teléfono 915 620 420, indicando el medicamento y la cantidad utilizada.
Si olvidó usar Femmyn 0,03 mg óvulos
- Durante el tratamiento diario de las tres primeras semanas:
Si olvida una dosis y no se da cuenta antes del día siguiente, no se preocupe por la dosis olvidada, y siga con el tratamiento.
- Durante el tratamiento de mantenimiento de dos veces por semana:
Si olvidó usar Femmyn el día previsto, adminístreselo lo antes posible y continúe con su pauta habitual.
Si interrumpe el tratamiento con Femmyn 0,03 mg óvulos
Aunque los síntomas mejoren considerablemente, debe continuar con el tratamiento hasta el final. Si quiere interrumpir o finalizar el tratamiento, consulte con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Las siguientes enfermedades se observan con mayor frecuencia en mujeres que utilizan medicamentos de THS que circulan en la sangre, en comparación con las mujeres que no utilizan THS. Estos riesgos se relacionan menos con los tratamientos administrados por vía vaginal, como Femmyn:
- Cáncer de ovario
- Coágulos de sangre en las venas de las piernas o de los pulmones (tromboembolismo venoso)
- Ictus
- Posiblemente pérdida de memoria, si la THS se inicia después de los 65 años de edad
Para mayor información sobre estos efectos adversos, ver la Sección 2.
Puede aparecer irritación local al inicio del tratamiento.
Efectos adversos frecuentes (de 1 a 10 de cada 100 usuarias)
- Quemazón vulvovaginal, prurito y dolor
- Molestias al orinar (disuria)
Efectos adversos poco frecuentes (de 1 a 10 de cada 1.000 usuarias)
- Secreción vaginal
- Molestias anorectales
Los siguientes efectos adversos se han notificado con otras THSs:
- Enfermedad de la vesícula biliar
- Varias afectaciones cutáneas:
- Decoloración de la piel, especialmente de la cara o del cuello, conocido como “parches de embarazo” (cloasma);
- Nódulos cutáneos rojos dolorosos (eritema nudoso);
- Erupción con enrojecimiento o llagas en forma de diana (eritema multiforme).
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Femmyn 0,03 mg óvulos
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la lámina después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25ºC.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Femmyn 0,03 mg óvulos
- El principio activo es estriol. Cada óvulo contiene 0,03 mg de estriol.
- Los demás componentes son butilhidroxitolueno, mono/di-(Z,R)-12-hidroxioctadeca-9-enoato de glicerol, grasa dura, éter cetoestearílico de macrogol.
Aspecto del producto y contenido del envase
Femmyn son óvulos blancos.
Femmyn se presenta en cajas de 10, 15, 20 ó 30 óvulos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización:
Kern Pharma, S.L.
Venus, 72 - Pol. Ind. Colón II
08228 Terrassa - Barcelona
España
Responsable de la fabricación:
Dr. Kade Pharmazeutische Fabrik GmbH
Rigistraße 2
12277 Berlin
Alemania
Fecha de la última revisión de este prospecto: Diciembre 2023
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)
http://www.aemps.gob.es/
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FEMMYN 0,03 MG ÓVULOSForma farmacéutica: SEMISOLIDO VAGINAL, 50 microgramos/gPrincipio activo: EstriolFabricante: Italfarmaco S.A.Requiere recetaForma farmacéutica: SEMISOLIDO VAGINAL, 50 microgramos/gPrincipio activo: EstriolFabricante: Italfarmaco S.A.Requiere recetaForma farmacéutica: OVULO/CAPSULA/COMPRIMIDO VAGINAL, 0,5 mg estriolPrincipio activo: EstriolFabricante: Aspen Pharma Trading LimitedRequiere receta
Médicos online para FEMMYN 0,03 MG ÓVULOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FEMMYN 0,03 MG ÓVULOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











