
DARUNAVIR TARBIS 400 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG

Cómo usar DARUNAVIR TARBIS 400 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Darunavir Tarbis 400 mg comprimidos recubiertos con película EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted,y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Darunavir Tarbis y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Darunavir Tarbis
- Cómo tomar Darunavir Tarbis
- Posibles efectos adversos
- Conservación de Darunavir Tarbis
- Contenido del envase e información adicional
1. Qué es Darunavir Tarbis y para qué se utiliza
¿Qué es Darunavir Tarbis?
Darunavir Tarbis contiene el principio activo darunavir. Darunavir Tarbis es un medicamento antirretroviral usado en el tratamiento de la infección por el Virus de la Inmunodeficiencia Humana (VIH). Pertenece a un grupo de medicamentos llamado inhibidores de la proteasa. Darunavir Tarbis reduce la cantidad de VIH presente en su cuerpo. Con ello, su sistema inmunitario mejorará y disminuirá el riesgo de sufrir enfermedades asociadas con la infección por el VIH.
¿Para qué se utiliza?
El comprimido de Darunavir Tarbis 400 miligramos se usa para tratar a los adultos y niños (a partir de 3 años de edad, con al menos 40 kilogramos de peso) infectados por el VIH y
- que no han usado antes medicamentos antirretrovirales.
- en ciertos pacientes que han usado antes medicamentos antirretrovirales (será determinado por su médico).
Este medicamento debe tomarse junto con una dosis baja de cobicistat o ritonavir y otros medicamentos contra el VIH. Su médico le expondrá la combinación de medicamentos más conveniente para usted.
2. Qué necesita saber antes de empezar a tomar Darunavir Tarbis
No tome Darunavir Tarbis
- si es alérgicoa darunavir o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6) o a cobicistat o a ritonavir.
- si padece problemas graves del hígado. Pregunte a su médico si usted no está seguro de la gravedad de su enfermedad hepática. Podría ser necesaria la realización de algunas pruebas adicionales.
No combine Darunavir Tarbis con ninguno de los medicamentos siguientes
Si está tomando cualquiera de estos fármacos, consulte a su médico para cambiar a otro medicamento.
Medicamento | Finalidad del medicamento |
Avanafilo | tratamiento de la disfunción eréctil |
Astemizolo terfenadina | tratamiento de los síntomas de la alergia |
Triazolamy midazolam(por vía oral) | ayudarle a dormir y/o aliviar la ansiedad |
Cisaprida | tratamiento de problemas de estómago |
Colchicina(si tiene problemas de riñón y/o hígado) | tratamiento de la gota o la fiebre Mediterránea familiar |
Lurasidona, pimozida, quetiapina o sertindole | tratamiento de problemas psiquiátricos |
Alcaloides del cornezuelo del centenocomo ergotamina, dihidroergotamina, ergometrinay metilergonovina | tratamiento de dolores de cabeza tipo migraña |
Amiodarona, bepridilo, dronedarona, ivabradina, quinidina, ranolazina | tratamiento de determinadas alteraciones cardiacas por ejemplo los latidos irregulares del corazón |
Lovastatina, simvastatina y lomitapida | reducir los niveles de colesterol |
Rifampicina | tratamiento de ciertas infecciones como la tuberculosis |
La combinación de medicamentos lopinavir/ritonavir | este medicamento contra el VIH pertenece a la misma clase que Darunavir Tarbis 400 mg comprimidos recubiertos con película EFG |
Elbasvir/grazoprevir | para tratar la infección de la hepatitis C |
Alfuzosina | tratamiento del aumento de tamaño de la próstata |
Sildenafilo | tratamiento de la presión arterial alta en la circulación pulmonar |
Dabigatrán, ticagrelor | para ayudar a parar la agregación de plaquetas durante el tratamiento de pacientes con antecedentes de infarto de corazón |
Naloxegol | para tratar el estreñimiento inducido por opioides |
Dapoxetina | para tratar la eyaculación precoz |
Domperidona | para tratar las náuseas y vómitos |
No combine este medicamento con productos que contienen hipérico o hierba de San Juan (Hypericum perforatum).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a tomar Darunavir Tarbis.
Darunavir no cura la infección por el VIH. Mientras esté tomando este medicamento aún puede transmitir el VIH a los demás, aunque el tratamiento antiviral eficaz reduzca el riesgo. Consulte a su médico sobre qué precauciones son necesarias para no infectar a otras personas.
Las personas que toman darunavir pueden desarrollar otras infecciones u otras enfermedades que se asocian con la infección por el VIH. Debe mantener un contacto regular con su médico.
Las personas que toman darunavir pueden desarrollar una erupción en la piel. No es frecuente que la erupción sea grave o potencialmente mortal. Por favor, consulte con su médico si desarrolla una erupción.
Los pacientes que toman darunavir y raltegravir (para la infección por el VIH), puede que presenten erupciones (generalmente de carácter leve o moderado) más frecuentemente que los pacientes que toman cualquiera de los dos medicamentos de forma separada.
Informe a su médico sobre su situación ANTES y DURANTE su tratamiento
Asegúrese de que comprueba los puntos siguientes e informe a su médico en caso de que alguno le aplique.
- Informe a su médico si ha sufrido alguna enfermedad del hígado, incluyendo la infección de la hepatitis B o C. Su médico valorará la gravedad de la enfermedad hepática antes de decidir si puede tomar este medicamento.
- Informe a su médico si tiene diabetes. Darunavir puede provocar un aumento de la concentración de azúcar en sangre.
- Informe a su médico inmediatamente si observa algún síntoma de infección(por ejemplo aumento de ganglios linfáticos y fiebre). En algunos pacientes con infección por el VIH avanzada y antecedentes de infecciones oportunistas pueden aparecer signos y síntomas de inflamación debidos a infecciones previas poco después de iniciar el tratamiento contra el VIH. Se cree que estos síntomas se deben a una mejoría de la respuesta inmune del organismo, que le permite combatir las infecciones que estaban presentes sin ningún síntoma aparente.
- Además de las infecciones oportunistas, también pueden aparecer trastornos autoinmunitarios (una afección que ocurre cuando el sistema inmunitario ataca tejido corporal sano) después de que usted haya empezado a tomar medicamentos para el tratamiento de su infección por VIH. Los trastornos autoinmunitarios pueden aparecer muchos meses después del inicio del tratamiento. Si observa cualquier síntoma de infección u otros síntomas como por ejemplo debilidad muscular, debilidad que empieza en las manos y pies y que asciende hacia el tronco del cuerpo, palpitaciones, temblor o hiperactividad, informe a su médico inmediatamente para recibir el tratamiento necesario.
- Informe a su médico si tiene hemofilia. Darunavir puede incrementar el riesgo de hemorragia.
- Informe a su médico si es alérgico a sulfonamidas(por ejemplo, usadas para el tratamiento de ciertas infecciones).
- Informe a su médico si advierte algún problema óseo o muscular.Algunos pacientes que utilizan tratamiento antirretroviral combinado pueden sufrir una osteopatía llamada osteonecrosis (muerte del tejido óseo provocada por la falta de riego sanguíneo en el hueso). Algunos de los muchos factores de riesgo de padecer esta enfermedad, entre otros, son la duración del tratamiento antirretroviral combinado, el empleo de corticoesteroides, el consumo de alcohol, la inmunodepresión grave y un mayor índice de masa corporal. Los signos de osteonecrosis son dolor, malestar y rigidez de las articulaciones (sobre todo de la cadera, las rodillas y los hombros) y dificultad para moverse. Si advierte alguno de estos síntomas, por favor, diríjase a su médico.
Población de edad avanzada
Darunavir solo ha sido usado en un número limitado de pacientes de 65 años o mayores. Si usted pertenece a este grupo de edad, por favor, hable con su médico para ver si puede usar este medicamento.
Niños y adolescentes
El comprimido de Darunavir Tarbis 400 miligramos no se debe utilizar en niños menores de 3 años de edad o con un peso menor a 40 kilogramos.
Toma de Darunavir Tarbis con otros medicamentos
Informe a su médico o farmacéutico si está tomando o ha tomado recientemente cualquier otro medicamento.
Algunos medicamentos no se deben combinarcon este medicamento. La lista puede consultarse en el apartado “No combine Darunavir Tarbis con ninguno de los medicamentos siguientes:”
En la mayoría de los casos, darunavir se puede combinar con medicamentos contra el VIH que pertenecen a otras clases [p.ej. INTIs (inhibidores de la transcriptasa inversa análogos de nucleósidos), INNTIs (inhibidores de la transcriptasa inversa no nucleósidos), antagonistas CCR5 e IFs (inhibidores de fusión)]. No se ha probado darunavir con cobicistat o ritonavir con todos los inhibidores de la proteasa (IPs) y no debe utilizarse con otros inhibidores de la proteasa del VIH. En algunos casos puede ser necesario cambiar la dosis de los otros medicamentos. Por lo tanto, si usted toma otros medicamentos anti-VIH informe siempre a su médico y siga cuidadosamente sus instrucciones sobre qué medicamentos se pueden combinar.
Los productos siguientes pueden reducir la eficacia de darunavir. Informe a su médico si toma:
- Fenobarbital, difenilhidantoína(para prevenir convulsiones)
- Dexametasona(corticoesteroide)
- Efavirenz(para la infección por VIH)
- Boceprevir(para tratar la infección de la hepatitis C)
- Rifapentina, rifabutina(medicamentos para tratar algunas infecciones como la tuberculosis)
- Saquinavir(para la infección por el VIH).
Darunavir también puede influir sobre los efectos de otros medicamentos. Informe a su médico si toma:
- Amlodipino, diltiazem, disopiramida, carvedilol, felodipino, flecainida, lidocaína, metoprolol, mexiletina, nifedipino, nicardipino, propafenona, timolol, verapamilo(para trastornos del corazón) porque los efectos terapéuticos o adversos de estos medicamentos se pueden ver aumentados.
- Apixabán, edoxabán, rivaroxaban, warfarina(para reducir la coagulación de la sangre) porque los efectos terapéuticos o adversos de estos medicamentos se pueden ver alterados; su médico puede que le haga análisis de sangre.
- Anticonceptivos hormonales basados en estrógenos y tratamientos hormonales de sustitución. Darunavir puede reducir su eficacia. Para el control de la natalidad, se recomiendan métodos anticonceptivos alternativos no hormonales.
- Etinilestradiol/drospirenona. Darunavir puede aumentar el riesgo de elevar los niveles de potasio por efecto de la drospirenona.
- Atorvastatina, pravastatina, rosuvastatina(para reducir el colesterol de la sangre). Puede haber un mayor riesgo de daño muscular. Su médico determinará qué tratamiento, para reducir el colesterol, le conviene más según sus circunstancias personales.
- Claritromicina(antibiótico)
- Ciclosporina, everolimus, tacrolimus, sirolimus(para inhibir el sistema inmunitario) porque los efectos terapéuticos o adversos de estos medicamentos se pueden ver aumentados. Su médico podría hacerle algunos análisis adicionales.
- Corticoesteroides, incluyendo betametasona, budesonida, fluticasona, mometasona, prednisona, triamcinolona.Estos medicamentos se usan para tratar alergias, asma, enfermedades inflamatorias del intestino, afecciones inflamatorias de los ojos, articulaciones y músculos, y otras afecciones inflamatorias. Si no se pueden usar alternativas, su uso solo debe efectuarse después de una evaluación clínica y con un estrecho seguimiento por parte de su médico para evaluar los efectos adversos de los corticoesteroides.
- Buprenorfina/naloxona(medicamentos para el tratamiento de la dependencia de opiáceos)
- Salmeterol(medicamento para el tratamiento del asma)
- Artemeter/lumefantrina(una combinación de medicamentos para tratar la malaria)
- Dasatinib, everolimus, irinotecan, nilotinib, vinblastina, vincristina(para tratar el cáncer)
- Sildenafilo, tadalafilo, vardenafilo(para la disfunción eréctil o para tratar un trastorno del corazón y pulmón llamado hipertensión arterial pulmonar)
- Glecaprevir/pibrentasvir, simeprevir(para tratar la infección de la hepatitis C)
- Fentanilo, oxicodona, tramadol(para tratar el dolor)
- Fesoterodina, solifenacina(para tratar los trastornos urológicos).
En ciertos casos, será necesario modificar la dosis de algunos medicamentos ya que al combinarse pueden verse afectados los efectos terapéuticos o adversos de estos o de darunavir.
Informe a su médico si toma:
- Alfentanilo(inyectable analgésico de acción fuerte y corta que se utiliza en los procedimientos quirúrgicos)
- Digoxina(para el tratamiento de ciertos trastornos cardiacos)
- Claritromicina(antibiótico)
- Itraconazol, isavuconazol, fluconazol, posaconazol, clotrimazol(para tratar las infecciones causadas por hongos). Voriconazol solo puede administrarse tras una evaluación médica.
- Rifabutina(contra infecciones bacterianas)
- Sildenafilo, vardenafilo, tadalafilo(para la disfunción eréctil o presión arterial alta en la circulación pulmonar)
- Amitriptilina, desipramina, imipramina, nortriptilina, paroxetina, sertralina, trazodona(para tratar la depresión yla ansiedad)
- Maraviroc(para tratar la infección por VIH)
- Metadona(para tratar la dependencia a narcóticos)
- Carbamazepina, clonazepam(para prevenir crisis epilépticas o para tratar ciertos tipos de dolor neuropático)
- Colchicina(para el tratamiento de la gota o la fiebre Mediterránea familiar)
- Bosentán(para el tratamiento de la presión arterial alta en la circulación pulmonar)
- Buspirona, clorazepato, diazepam, estazolam, flurazepam, midazolam cuando se administra en inyección, zolpidem(agentes sedantes)
- Perfenazina, risperidona, tioridazina(para tratar condiciones psiquiátricas)
- Metformina(para tratar diabetes tipo 2).
Esta noes una lista completa de medicamentos. Informe a su médico sobre todoslos medicamentos que usted esté tomando.
Toma de Darunavir Tarbis con alimentos y bebidas
Ver sección 3 “Cómo tomar Darunavir Tarbis”
Embarazo y lactancia
Informe a su médico inmediatamente si está embarazada, está planeando quedarse embarazada o está dando el pecho a su bebé. Las madres embarazadas o en periodo de lactancia, no deben tomar darunavir con ritonavir a menos que su médico se lo indique específicamente. Las embarazadas o madres en periodo de lactancia no deben tomar darunavir con cobicistat.
Se recomienda que las mujeres infectadas por VIH no den el pecho a sus hijos puesto que existe la posibilidad, de que los hijos se infecten por el VIH a través de la leche, como por los efectos desconocidos del medicamento en los niños.
Conducción y uso de máquinas
No maneje herramientas o máquinas ni conduzca si sufre mareos después de tomar este medicamento.
3. Cómo tomar Darunavir Tarbis
Siga exactamente las instrucciones de administración de este medicamento contenidas en este prospecto o las indicadas por su médico, farmacéutico o enfermero. En caso de duda pregunte a su médico, farmacéutico o enfermero. No deje de tomar darunavir ni cobicistat o ritonavir sin consultar antes a su médico aunque se sienta mejor.
Después de iniciado el tratamiento, no se debe cambiar la dosis o forma de la dosis o interrumpir el tratamiento sin instrucciones del médico.
Los comprimidos de Darunavir Tarbis 400 miligramos se usan solo para obtener la pauta posológica de 800 miligramos una vez al día.
Dosis para adultos que no han tomado antes medicamentos antirretrovirales (serán determinadas por su médico)
La dosis normal de darunavir es 800 miligramos (2 comprimidos de 400 miligramos de darunavir o 1 comprimido que contiene 800 miligramos de darunavir) una vez al día.
Debe tomar darunavir cada día y siempre en combinación con 150 miligramos de cobicistat o 100 miligramos de ritonavir y con alimentos. Darunavir no actúa adecuadamente sin cobicistat o ritonavir y alimentos. Antes de tomar los comprimidos de darunavir y cobicistat o ritonavir, debe ingerir alimento 30 minutos antes. El tipo de alimento no es importante. No interrumpa el tratamiento con darunavir ni con cobicistat o ritonavir sin consultar antes a su médico aunque se sienta mejor.
Instrucciones para adultos
- Tome dos comprimidos de 400 miligramos a la vez, una vez al día, todos los días.
- Tome darunavir siempre junto con 150 miligramos de cobicistat o 100 miligramos de ritonavir.
- Tome los comprimidos con alimentos.
- Trague los comprimidos con una bebida, que puede ser agua o leche.
- Tome los otros medicamentos para el VIH usados en combinación con darunavir y cobicistat o ritonavir como su médico le recomiende.
- La suspensión oral de darunavir 100 miligramos por mililitro ha sido desarrollada para su uso en niños, pero en algunos casos también puede ser utilizada en adultos.
Dosis para adultos que han tomado antes medicamentos antirretrovirales (serán determinadas por su médico)
La dosis es:
- 800 miligramos de darunavir (2 comprimidos que contienen 400 miligramos de darunavir o 1 comprimido que contiene 800 miligramos de darunavir) junto con 150 miligramos de cobicistat o 100 miligramos de ritonavir una vez al día.
O
- 600 miligramos de darunavir (1 comprimido que contiene 600 miligramos de darunavir) junto con 100 miligramos de ritonavir dos veces al día.
Por favor hable con su médico sobre qué dosis es la correcta para usted.
Dosis para niños a partir de 3 años de edad, con más de 40 kilogramos que no han tomado antes medicamentos antirretrovirales (el médico de su hijo la determinará)
- La dosis habitual de darunavir es 800 miligramos (2 comprimidos que contienen
400 miligramos de darunavir o 1 comprimido que contiene 800 miligramos de darunavir) junto con 100 miligramos de ritonavir una vez al día.
Dosis para niños a partir de 3 años de edad, con más de 40 kilogramos que han tomado antes medicamentos antirretrovirales (el médico de su hijo la determinará)
La dosis es:
- 800 miligramos de darunavir (2 comprimidos que contienen 400 miligramos de darunavir o 1 comprimido que contiene 800 miligramos de darunavir) junto con 100 miligramos de ritonavir una vez al día.
O
- 600 miligramos de darunavir (1 comprimido que contiene 600 miligramos de darunavir) junto con 100 miligramos de ritonavir dos veces al día.
Por favor, hable con su médico sobre qué dosis es la correcta para usted.
Instrucciones para niños a partir de 3 años de edad, con más de 40 kilogramos
- Tome 800 miligramos de darunavir (2 comprimidos que contienen 400 miligramos de darunavir o 1 comprimido que contiene 800 miligramos de darunavir) a la misma hora una vez al día todos los días.
- Tome darunavir siempre junto con 100 miligramos de ritonavir.
- Tome los comprimidos con alimentos.
- Trague los comprimidos con un líquido como el agua o la leche.
- Tome el resto de medicamentos utilizados en combinación con darunavir y ritonavir según le haya indicado su médico.
Extracción del tapón resistente a los niños
El frasco de plástico tiene un cierre de seguridad resistente a niños y se abre de la forma siguiente:
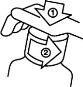 Empuje el tapón de plástico hacia abajo, girándolo al mismo tiempo contra el sentido de las agujas del reloj.
Empuje el tapón de plástico hacia abajo, girándolo al mismo tiempo contra el sentido de las agujas del reloj.- Saque el tapón desenroscando.
Si toma más Darunavir Tarbis del que debe
Informe inmediatamente a su médico, farmacéutico o enfermero.
En caso de sobredosis o ingestión accidental, consulte a su médico o farmacéutico o llame al Servicio de Infomación Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar Darunavir Tarbis
Si se da cuenta en las 12 horas siguientes, tome los comprimidos inmediatamente. Siempre toma la dosis con cobicistat o ritonavir y con alimento. Si se da cuenta después de 12 horas, omita esa toma y haga la siguiente de la forma acostumbrada. No tome una dosis doble para compensar las dosis olvidadas.
No deje de tomar Darunavir Tarbis sin hablar antes con su médico
Los medicamentos contra el VIH pueden hacer que se sienta mejor. Incluso aunque se sienta mejor, no deje de tomar este medicamento. Consulte primero a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Durante el tratamiento del VIH puede haber un aumento en el peso y en los niveles de glucosa y lípidos en la sangre. Esto puede estar en parte relacionado con la recuperación de la salud y con el estilo de vida y en el caso de los lípidos en la sangre, algunas veces a los medicamentos para el VIH por sí mismos. Su médico le controlará estos cambios.
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico si desarrolla alguno de los siguientes efectos adversos.
Se han notificado casos de problemas en el hígado que ocasionalmente pueden ser graves. Su médico le hará un análisis de sangre antes de que empiece el tratamiento con darunavir. Si tiene una infección crónica causada por la hepatitis B o C, su médico comprobará a menudo sus analíticas de sangre dado que existe una mayor probabilidad de desarrollar problemas en el hígado. Hable con su médico sobre los signos y síntomas de los problemas en el hígado. Estos pueden incluir que la piel y el blanco de los ojos se amarillee, oscurecimiento (color té) de la orina, heces de color pálido (movimientos del intestino), náuseas, vómitos, pérdida de apetito, o dolor, sensación de dolor o molestias en el lado derecho por debajo de sus costillas.
Erupción de la piel (más frecuente cuando se utiliza en combinación con raltegravir), picores. La erupción de la piel suele ser de leve a moderada. Una erupción de la piel también puede ser un síntoma de una situación rara y grave. Por eso, es importante que hable con su médico si presenta una erupción. Su médico le aconsejará sobre cómo controlar los síntomas o si debe interrumpir darunavir.
Otros efectos adversos graves fueron diabetes (frecuente) e inflamación del páncreas (poco frecuente). Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes)
- diarrea.
Efectos adversos frecuentes(pueden afectar a hasta 1 de cada 10 pacientes)
- vómitos, náuseas, dolor o distensión abdominal, dolor en la parte alta del abdomen (dispepsia), flatulencia
- dolor de cabeza, cansancio, mareos, somnolencia, sensación de adormecimiento, entumecimiento, hormigueo o dolor en las manos o en los pies, pérdida de fuerza, dificultad para quedarse dormido.
Efectos adversos poco frecuentes(pueden afectar a hasta 1 de cada 100 pacientes)
- dolor en el pecho, cambios en el electrocardiograma, movimientos rápidos del corazón
- disminución o anormal sensibilidad en la piel, hormigueo, trastorno de atención, pérdida de memoria, dificultad para mantener el equilibrio
- dificultad respiratoria, tos, hemorragia nasal, irritación de garganta
- inflamación del estómago o boca, ardor de estómago, arcadas, boca seca, molestias de abdomen, estreñimiento, eructar
- insuficiencia renal, cálculos renales, dificultad al orinar, orina excesiva o frecuente, a veces de noche
- urticaria, hinchazón grave de la piel y otros tejidos (sobre todo, los labios o los ojos), eczema, sudoración excesiva, sudores nocturnos, alopecia, acné, piel escamada, coloración de las uñas
- dolor muscular, calambres musculares o debilidad, dolores en las extremidades, osteoporosis
- función de glándula tiroides reducida. Esto se puede ver en un análisis de sangre.
- hipertensión (aumento de la presión arterial), rubor
- ojos rojos o secos
- fiebre, hinchazón de las extremidades inferiores por la retención de líquidos, malestar, irritabilidad, dolor
- síntomas de infección, herpes simple
- disfunción eréctil, aumento de tamaño de las mamas
- problemas para conciliar el sueño, somnolencia, depresión, ansiedad, sueños anormales, disminución del deseo sexual.
Efectos adversos raros(que pueden afectar a hasta 1 de cada 1.000 pacientes)
- una reacción llamada DRESS [erupción grave, que puede ir acompañada de fiebre, cansancio, hinchazón de la cara o ganglios linfáticos, aumento de esosinófilos (un tipo de célula blanca de la sangre), daños en el hígado, riñón o pulmón]
- infarto de miocardio, movimientos lentos del corazón, palpitaciones
- alteración visual
- escalofríos, sensación rara
- una sensación de confusión o desorientación, estado de ánimo alterado, agitación
- desmayo, crisis epiléptica, cambios o pérdida del gusto
- úlceras en la boca, vomitar sangre, inflamación de los labios, labios secos, lengua con sarro
- secreción de la nariz
- lesiones en la piel, sequedad de la piel
- rigidez muscular o en las articulaciones, dolores articulares con o sin inflamación
- cambios en alguno de los valores de las células de la sangre o bioquímica. Estos cambios se pueden ver en los análisis de sangre y/u orina. Su médico se los explicará. Por ejemplo: aumento en algunas células blancas de la sangre.
Algunos efectos adversos son típicos de los medicamentos contra el VIH que pertenecen a la misma familia que darunavir. Estos son:
- dolores musculares, sensibilidad o debilidad. En raras ocasiones, estos trastornos musculares pueden ser graves.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto.
También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Darunavir Tarbis
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el frasco, después de CAD. La fecha de caducidad se refiere al último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGREde la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Darunavir Tarbis 400 mg comprimidos recubiertos con película EFG
- El principio activo es darunavir. Cada comprimido contiene 400 mg de darunavir.
- Los demás componentes son sílice coloidal anhidra (E551), celulosa microcristalina silificada (E460), crospovidona (E1202) y esterarato de magnesio (E470b). El recubrimiento del comprimido contiene alcohol polivinílico - parcialmente hidrolizado, macrogol, dióxido de titanio (E171), talco (E553b) y óxido de hierro amarillo (E172).
Aspecto de Darunavir Tarbis 400 mg comprimidos recubiertos con película EFG y contenido del envase
Comprimidos ovalados, biconvexos recubiertos con película color amarillo, de aprox. 15,7 mm de longitud y 7,9 mm de anchura, grabados con una 'V' en una cara, y con un '4' en la otra.
Envase de blísteres:
Blísteres de aluminio en caja de cartón. Cada caja contiene dosis unitarias de comprimidos recubiertos con película en blísteres de 30 x 1, 60 x 1 o 90 x 1 unidades.
Frasco de plástico:
Frasco de polipropileno de alta densidad (HDPE) blanco opaco en una caja de cartón. El frasco contiene un recipiente con desecante de gel de sílice. Este recipiente debe dejarse dentro del frasco para la protección de los comprimidos y no debe tragarse.
Cada caja contiene 60 comprimidos recubiertos con película.
Darunavir Tarbis también está disponible en comprimidos recubiertos con película de 600 mg y 800 mg.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Tarbis Farma S.L.
Gran Vía Carlos III, 94
08028 Barcelona
España
Responsable de la fabricación
Pharmadox Healthcare Ltd.
KW20A Kordin Industrial Park
PLA 3000 Paola
Malta
Amarox Pharma B.V.
Rouboslaan 32
2252 TR Voorschoten
Países Bajos
Este medicamento está autorizado en los Estados Miembros del EEE bajo los siguientes nombres:
Países Bajos: Darunavir Amarox 400 mg filmomhulde tabletten
Suecia: Darunavir Amarox 400 mg Filmdragerade tabletter
Alemania: Darunavir Amarox 400 mg Filmtabletten
España: Darunavir Tarbis 400 mg comprimidos recubiertos con película EFG
Fecha de la última revisión de este prospecto:Marzo 2020
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a DARUNAVIR TARBIS 400 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFGForma farmacéutica: COMPRIMIDO, 600 mgPrincipio activo: DarunavirFabricante: Aurovitas Spain, S.A.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 800 mgPrincipio activo: DarunavirFabricante: Aurovitas Spain, S.A.U.Requiere recetaForma farmacéutica: COMPRIMIDO, 400 mgPrincipio activo: DarunavirFabricante: Krka D.D. Novo MestoRequiere receta
Médicos online para DARUNAVIR TARBIS 400 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de DARUNAVIR TARBIS 400 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















