SANDIMMUN NEORAL 100 mg/ml SOLUCION ORAL

Cómo usar SANDIMMUN NEORAL 100 mg/ml SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
Sandimmun Neoral 100mg/ml solución oral
ciclosporina
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Sandimmun Neoral y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Sandimmun Neoral
- Cómo tomar Sandimmun Neoral
- Posibles efectos adversos
- Conservación de Sandimmun Neoral
- Contenido del envase e información adicional
1. Qué es Sandimmun Neoral y para qué se utiliza
Qué es Sandimmun Neoral
El nombre de su medicamento es Sandimmun Neoral. Contiene el principio activo ciclosporina. Pertenece a un grupo de medicamentos conocido como inmunosupresores. Estos medicamentos se utilizan para disminuir las reacciones inmunológicas del organismo.
Para qué se utiliza Sandimmun Neoral y como funciona
- Si usted ha sido sometido a un trasplante de órgano, de médula ósea y células madre,la función de Sandimmun Neoral es la de controlar el sistema inmune de su organismo. Sandimmun Neoral previene el rechazo de órganos trasplantados frenando el desarrollo de ciertas células que normalmente atacarían al tejido trasplantado.
- Si usted padece una enfermedad autoinmune,en la cual la respuesta inmune de su organismo ataca a las propias células de su organismo, Sandimmun Neoral frena esta reacción inmune. Estas enfermedades incluyen problemas en los ojos que pueden afectar a su visión (uveítis endógena, incluida uveítis de Behçet), casos graves de ciertas enfermedades de la piel (dermatitis atópica, o eczema, y psoriasis), artritis reumatoide grave y una enfermedad renal denominada síndrome nefrótico.
2. Qué necesita saber antes de empezar a tomar Sandimmun Neoral
Si usted está tomando Sandimmun Neoral después de un trasplante, sólo se lo puede haber prescrito un médico con experiencia en trasplantes y/o enfermedades autoinmunes.
Las recomendaciones en este prospecto pueden variar dependiendo de si usted está tomando el medicamento para un trasplante o para una enfermedad autoinmune.
Siga detalladamente todas las instrucciones de su médico. Pueden ser diferentes de la información general contenida en este prospecto.
No tomeSandimmun Neoral:
- si es alérgico a la ciclosporina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- con medicamentos que contienen Hypericum perforatum (hierba de San Juan).
- con medicamentos que contienen dabigatrán etexilato (utilizado para evitar coágulos de sangre tras una operación) o bosentán y aliskirén (utilizados para bajar la presión sanguínea alta).
Si esto le aplica a usted, no tome Sandimmun Neoral e informe a su médico. Si no está seguro, consulte con su médico antes de tomar Sandimmun Neoral.
Advertencias y precauciones
Antes y durante el tratamiento con Sandimmun Neoral, informe a su médico inmediatamente:
- si presenta cualquier signo de infección, tal como fiebre o dolor de garganta. Sandimmun Neoral suprime el sistema inmune y puede además influir sobre la capacidad de su organismo para luchar contra las infecciones.
- si tiene problemas de hígado.
- si tiene problemas de riñón. Su médico le realizará análisis de sangre con regularidad y podría cambiar su dosis en caso necesario.
- si tiene alta la presión sanguínea. Su médico controlará su presión sanguínea con regularidad y podría administrarle un medicamento para bajar la presión arterial en caso que fuese necesario.
- si presenta niveles bajos de magnesio en su organismo. Su médico puede administrarle suplementos de magnesio, especialmente justo después de su operación si usted ha sido sometido a un trasplante.
- si tiene niveles altos de potasio en sangre.
- si sufre de gota.
- si necesita recibir una vacuna.
Si antes o durante el tratamiento con Sandimmun Neoral sufre alguna de las situaciones anteriores, informe a su médico inmediatamente.
Protección solar y luz solar
Sandimmun Neoral suprime su sistema inmune. Esto puede incrementar el riesgo de desarrollar cáncer, principalmente de la piel y del sistema linfático. Por lo tanto, debe limitar su exposición al sol y a los rayos UV de la siguiente forma:
- Llevando ropa protectora adecuada.
- Aplicando frecuentemente un filtro solar con un factor de protección alto.
Consulte con su médico antes de tomar Sandimmun Neoral:
- si tiene o ha tenido problemas relacionados con el alcohol.
- si padece de epilepsia.
- si tiene cualquier problema en el hígado.
- si está embarazada.
- si está en periodo de lactancia.
- si este medicamento se prescribe a un niño.
Si alguna de estas situaciones le aplica a usted (o no está seguro), informe a su médico antes de tomar Sandimmun Neoral. Esto es debido a que este medicamento contiene alcohol (ver sección, “Sandimmun Neoral contiene etanol”, más adelante).
Monitorización durante su tratamiento con Sandimmun Neoral
Su médico controlará:
- los niveles de ciclosporina en sangre, especialmente si ha sido sometido a un trasplante,
- su presión sanguíneaantes de iniciar el tratamiento y regularmente durante el mismo,
- como están funcionando su hígado y riñones,
- su nivel de lípidos en sangre(grasas).
Si tiene alguna pregunta de como funciona Sandimmun Neoral o de por qué se le ha prescrito este medicamento, consulte con su médico.
Además, si está tomando Sandimmun Neoralpara una enfermedad distinta de un trasplante(uveítis intermediaria o posterior y uveítis de Behçet, dermatitis atópica, artritis reumatoide grave o síndrome nefrótico), no tome Sandimmun Neoral:
- si tiene problemas de riñón (excepto para síndrome nefrótico).
- si tiene una infección que no está controlada con medicación.
- si tiene algún tipo de cáncer.
- si tiene alta la presión sanguínea (hipertensión) que no está controlada con medicamentos. Si sufre presión sanguínea alta durante el tratamiento y no se puede controlar, su médico debe interrumpir el tratamiento con Sandimmun Neoral.
Si alguna de estas situaciones le aplica a usted, no tome Sandimmun Neoral. Si no está seguro, consulte con su médico o farmacéutico antes de tomar Sandimmun Neoral.
Si a usted se le está tratando la uveítis de Behçet, su médico le controlará cuidadosamente, en especial si presenta síntomas neurológicos (por ejemplo: olvidos frecuentes, cambios en la personalidad observado con el tiempo, trastornos psiquiátricos o del estado de ánimo, sensación de ardor en las extremidades, disminución de la sensibilidad en las extremidades, sensación de hormigueo en las extremidades, debilidad de las extremidades, alteraciones motoras, dolor de cabeza con o sin náuseas y vómitos, trastornos de la vista que incluyen movimientos limitados del globo ocular).
Su médico le controlará estrechamente si usted es una persona de edad avanzada y le están tratando para psoriasis o dermatitis atópica. Si le han prescrito Sandimmun Neoral para tratar su psoriasis o dermatitis atópica, no se debe exponer a los rayos UVB o fotoquimioterapia durante el tratamiento.
Niños y adolescentes
Sandimmun Neoral no se debe administrar a niños para otras indicaciones distintas del trasplante, excepto para el tratamiento del síndrome nefrótico.
Población de edad avanzada (a partir de 65años de edad)
Existe experiencia limitada con Sandimmun Neoral en pacientes de edad avanzada. Su médico debe controlar como funcionan sus riñones. Si usted es mayor de 65 años y padece de psoriasis o dermatitis atópica, únicamente se debe tratar con Sandimmun Neoral si su estado es especialmente grave.
Otros medicamentos y Sandimmun Neoral
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
En particular informe a su médico o farmacéutico si está tomando cualquiera de los siguientes medicamentos antes o durante el tratamiento con Sandimmun Neoral:
- Medicamentos que pueden afectar sus niveles de potasio. Estos incluyen medicamentos que contienen potasio, suplementos de potasio, diuréticos ahorradores de potasio, y algunos medicamentos que bajan su presión sanguínea.
- Metotrexato. Se utiliza para tratar tumores, psoriasis grave y artritis reumatoide grave.
- Medicamentos que pueden aumentar o disminuir los niveles de ciclosporina (el principio activo de Sandimmun Neoral) en su sangre. Su médico podría comprobar el nivel de ciclosporina en su sangre cuando inicie o interrumpa el tratamiento con otros medicamentos.
- Los medicamentos que pueden aumentar el nivel de ciclosporina en su sangre incluyen: antibióticos (tales como eritromicina o azitromicina), antifúngicos (voriconazol, itraconazol), medicamentos utilizados para los trastornos del corazón o presión sanguínea alta (diltiazem, nicardipino, verapamilo, amiodarona), metoclopramida (utilizada para detener los vómitos), anticonceptivos orales, danazol (utilizado para tratar los trastornos menstruales), medicamentos utilizados para tratar la gota (alopurinol), ácido cólico y derivados (utilizado para tratar los cálculos biliares), inhibidores de la proteasa utilizados para tratar el VIH, imatinib (utilizado para tratar la leucemia o tumores), colchicina, telaprevir (utilizado para tratar la hepatitis C), cannabidiol (su uso incluye, entre otros, el tratamiento de las crisis epilépticas).
- Los medicamentos que pueden disminuir el nivel de ciclosporina en su sangre incluyen: barbitúricos (utilizados para ayudarle a dormir), ciertos medicamentos anticonvulsivantes (tales como carbamazepina o fenitoína), octreótida (utilizada para tratar la acromegalia o los tumores neuroendocrinos en el intestino), medicamentos antibacterianos utilizados para tratar la tuberculosis, orlistat (utilizado para ayudar a perder peso), medicamentos a base de plantas medicinales que contienen hierba de San Juan, ticlopidina (utilizado después de un accidente cerebrovascular), ciertos medicamentos que bajan la presión sanguínea (bosentán), y terbinafina (un medicamento antifúngico, utilizado para tratar infecciones de los dedos de los pies y las uñas).
- Medicamentos que pueden afectar sus riñones. Estos incluyen: medicamentos antibacterianos (gentamicina, tobramicina, ciprofloxacino), medicamentos antifúngicos que contienen anfotericina B, medicamentos utilizados para infecciones del tracto urinario que contienen trimetoprim, medicamentos para cáncer que contienen melfalán, medicamentos utilizados para disminuir la cantidad de ácido en su estómago (inhibidores de la secreción ácida del tipo antagonistas del receptor H2), tacrolimús, analgésicos (medicamentos antiinflamatorios no esteroideos como diclofenaco), medicamentos de ácido fíbrico (utilizados para disminuir la cantidad de grasa en la sangre).
- Nifedipino. Se utiliza para tratar la presión sanguínea alta y el dolor en el pecho. Podría tener las encías inflamadas que podrían crecer sobre sus dientes, si está tomando nifedipino durante su tratamiento con ciclosporina.
- Digoxina (utilizada para tratar trastornos cardiacos), medicamentos que reducen el colesterol (inhibidores de la HMG-CoA reductasa también llamados estatinas), prednisolona, etopósido (utilizado para tratar el cáncer), repaglinida (un medicamento antidiabético oral), inmunosupresores (everolimús, sirolimús), ambrisentán y medicamentos contra el cáncer específicos denominados antraciclinas (tal como doxorrubicina).
- Micofenolato de sodio o micofenolato de mofetilo (un inmunosupresor) y eltrombopag (utilizado para tratar trastornos hemorrágicos).
Si alguna de estas situaciones le aplica a usted (o no está seguro), informe a su médico o farmacéutico antes de tomar Sandimmun Neoral.
Toma de Sandimmun Neoral con alimentos y bebidas
No tome Sandimmun Neoral con pomelo o zumo de pomelo. Esto es debido a que puede afectar al funcionamiento de Sandimmun Neoral.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de tomar este medicamento.
- Informe a su médico si está embarazada o tiene intención de quedarse embarazada.La experiencia con Sandimmun Neoral en mujeres embarazadas es limitada. En general, Sandimmun Neoral no se debe administrar durante el embarazo. Si es necesario para usted tomar este medicamento, su médico le comentará los beneficios y riesgos potenciales de tomarlo durante el embarazo.
- Informe a su médico si está en periodo de lactancia.La lactancia no está recomendada durante el tratamiento con Sandimmun Neoral. Esto es debido a que la ciclosporina, el principio activo, pasa a la leche materna y podría afectar a su niño.
Hepatitis C
Informe a su médico si tiene hepatitis C. Su función hepática puede cambiar con el tratamiento de la hepatitis C y esto puede afectar a los niveles de ciclosporina en la sangre. Es posible que su médico necesite controlar de cerca los niveles en sangre de ciclosporina y hacer ajustes de la dosis después de iniciar el tratamiento para la hepatitis C.
Conducción y uso de máquinas
Puede sentirse somnoliento, desorientado o tener visión borrosa después de tomar Sandimmun Neoral. Tenga cuidado al conducir u operar maquinaria mientras esté tomando Sandimmun Neoral hasta que sepa cómo le afecta.
Sandimmun Neoral contieneetanol
Sandimmun Neoral contiene 94,70 mg de alcohol (etanol) por ml que se corresponde con 12,0 % v/v. Una dosis de 500 mg de Sandimmun Neoral contiene 500 mg de etanol, equivalente a cerca de 13 ml de cerveza o 5 ml de vino. La pequeña cantidad de alcohol en este medicamento no tendrá ningún efecto perceptible.
Sandimmun Neoral contiene aceite de ricino
Sandimmun Neoral puede producir molestias de estómago y diarrea porque contiene aceite de ricino.
Sandimmun Neoral contiene propilenglicol
Este medicamento contiene 94,70 mg de propilenglicol en cada ml de la solución oral.
Si el bebé tiene menos de 4 semanas de edad, consulte a su médico o farmacéutico, en particular si al bebé se le han administrado otros medicamentos que contengan propilenglicol o alcohol.
3. Cómo tomar Sandimmun Neoral
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
No tome más de la dosis recomendada.
Su médico ajustará cuidadosamente la dosis de este medicamento a sus necesidades individuales. Demasiada cantidad de este medicamento puede afectar a sus riñones. Se le harán análisis de sangre regulares y visitas al hospital, especialmente después del trasplante. Esto le permitirá consultar con su médico acerca de su tratamiento y de cualquier problema que pueda tener.
Qué cantidad de Sandimmun Neoral debe tomar
Su médico le indicará la dosis correcta de Sandimmun Neoral para usted. Esto depende de su peso corporal y de para qué está tomando este medicamento. Asimismo, su médico le informará con qué frecuencia debe tomar su medicamento.
- En adultos:
Trasplante de órganos, médula ósea y células madre
- La dosis diaria total normalmente se encuentra entre 2 mg y 15 mg por kilogramo de peso corporal. Ésta se divide en dos dosis.
- Normalmente, las dosis altas se utilizan antes y justo después de su trasplante. Las dosis más bajas se utilizan una vez su órgano trasplantado o médula ósea están estabilizados.
- Su médico le ajustará la dosis a una que sea ideal para usted. Para ello, puede que su médico necesite hacerle algún análisis de sangre.
Uveítis endógena
- La dosis diaria total normalmente se encuentra entre 5 mg y 7 mg por kilogramo de peso corporal. Ésta se divide en dos dosis.
Síndrome nefrótico
- La dosis diaria total para adultos normalmente es de 5 mg por kilogramo de peso corporal. Ésta se divide en dos dosis. En pacientes con alteraciones renales, la primera dosis diaria que tome no debe ser superior a 2,5 mg por kilogramo de peso corporal.
Artritis reumatoide grave
- La dosis diaria total normalemnte se encuentra entre 3 mg y 5 mg por kilogramo de peso corporal. Ésta se divide en dos dosis.
Psoriasis y dermatitis atópica
- La dosis diaria total normalmente se encuentra entre 2,5 mg y 5 mg por kilogramo de peso corporal. Ésta se divide en dos dosis.
- En niños:
Síndrome nefrótico
- La dosis diaria total para niños normalmente es de 6 mg por kilogramo de peso corporal. Ésta se divide en dos dosis. En pacientes con alteraciones renales, la primera dosis diaria que tome no debe ser superior a 2,5 mg por kilogramo de peso corporal.
Siga exactamente las instrucciones de su médico y no cambie nunca la dosis usted mismo, aunque se encuentre bien.
Conversión de Sandimmun a Sandimmun Neoral
Puede que usted ya haya estado tomando otro medicamento denominado Sandimmun cápsulas de gelatina blanda o Sandimmun solución oral. Puede que su médico decida cambiarle a Sandimmun Neoral solución oral.
- Todos estos medicamentos contienen ciclosporina como principio activo.
- Sandimmun Neoral es una formulación diferente y mejorada de ciclosporina comparado con Sandimmun. La ciclosporina se absorbe mejor en la sangre con Sandimmun Neoral y es menos probable que se vea afectada por la ingesta del medicamento con alimentos. Esto significa que los niveles de ciclosporina en la sangre permanecen más constantes con Sandimmun Neoral que con Sandimmun.
Si su médico le cambia de Sandimmun a Sandimmun Neoral:
- No vuelva a tomar Sandimmun a no ser que su médico se lo indique.
- Tras la conversión de Sandimmun a Sandimmun Neoral, su médico le controlará más estrechamente durante un corto periodo de tiempo. Esto es debido al cambio en como la ciclosporina se absorbe en la sangre. Su médico se asegurará de que tome la dosis correcta de acuerdo con sus necesidades individuales.
- Puede presentar algunos efectos adversos. Si esto ocurre, informe a su médico o farmacéutico. Puede ser necesario reducir su dosis. Nunca reduzca su dosis usted mismo, a menos que se lo indique un médico.
Si su médico le cambia de una formulación oral de ciclosporina a otra
Después de que usted cambie de una formulación oral de ciclosporina a otra:
- Su médico le controlará más estrechamente durante un corto periodo de tiempo.
- Puede presentar algunos efectos adversos. Si esto ocurre, informe a su médico o farmacéutico. Puede ser necesario modificar su dosis. Nunca modifique su dosis usted mismo, a menos que se lo indique un médico.
Cuándo tomar Sandimmun Neoral
Tome Sandimmun Neoral en el mismo momento cada día. Esto es muy importante si usted ha sido sometido a un trasplante.
Cómo tomar Sandimmun Neoral
Su dosis diaria se debe administrar siempre dividida en 2 dosis.
- Para su uso inicial, siga los pasos del 1 al 9.
- Para su uso posterior, siga los pasos del 5 al 9.
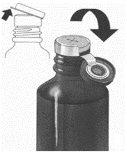
Instrucciones para empezar un nuevo frasco de Sandimmun Neoral solución oral
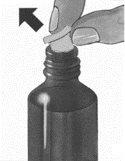
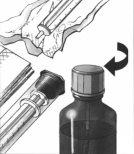
1. | Levante la tapa en el centro del anillo del cierre metálico. |
|
2. | Arranque por completo el anillo de cierre. |
|
3. | Retire el tapón gris y deséchelo. |
|
4. | Introduzca y presione con fuerza el tubo con tapón blanco en el cuello del frasco. |
|
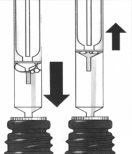
Medición de la dosis | ||
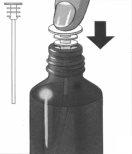
5. | Escoja la jeringa dependiendo de cuanto medicamento necesita medir:
Introduzca la punta de la jeringa en el tapón blanco. |
|
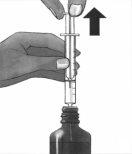
6. | Levante el émbolo hasta que extraiga la cantidad correcta de medicamento.
|
|
7. | Presione hacia abajo y levante el émbolo varias veces.
Asegúrese de que la cantidad correcta de medicamento está contenida en la jeringa. Posteriormente, retire la jeringa del frasco. |
|
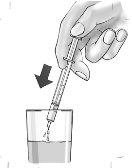
8. | Expulse el medicamento de la jeringa a un vaso de vidrio con algo de líquido, preferiblemente zumo de manzana.
|
|
9. | Después de su uso, limpie sólo el exterior de la jeringa con un paño seco.
|
|
Cuánto tiempo tomar Sandimmun Neoral
Su médico le indicará durante cuanto tiempo necesita tomar Sandimmun Neoral. Esto depende de si lo está tomando después de un trasplante o para el tratamiento de enfermedades graves de la piel, artritis reumatoide, uveítis o síndrome nefrótico. En el caso de erupción cutánea grave, el tratamiento normalmente dura 8 semanas.
Continúe tomando Sandimmun Neoral durante todo el tiempo que su médico se lo indique.
Si tiene dudas acerca del tiempo que debe tomar Sandimmun Neoral, consulte con su médico o farmacéutico.
Si toma más Sandimmun Neoral del que debe
Si accidentalmente toma una dosis excesiva del medicamento, informe a su médico inmediatamente, o acuda a la unidad de urgencias del hospital más cercano. Puede necesitar atención médica.
También puede llamar al Servicio de Información Toxicológica teléfono 91 562 04 20, indicando el medicamento y la cantidad tomada.
Si olvidó tomar Sandimmun Neoral
- Si olvidó tomar una dosis, tómela tan pronto se acuerde. Sin embargo, si está cerca de la siguiente dosis, salte la dosis olvidada. Luego continúe como anteriormente.
- No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Sandimmun Neoral
No interrumpa el tratamiento con Sandimmun Neoral a menos que su médico se lo indique.
Continúe tomando Sandimmun Neoral incluso si se encuentra bien. La interrupción del tratamiento con Sandimmun Neoral puede aumentar el riesgo de rechazo de su órgano trasplantado.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Algunos efectos adversos pueden ser graves
Informe a su médico inmediatamentesi nota alguno de los siguientes efectos adversos graves:
- Al igual que otros medicamentos que actúan sobre el sistema inmune, la ciclosporina puede influir sobre la capacidad de su organismo para combatir ciertas infecciones y puede causar tumores u otras enfermedades malignas, especialmente de la piel. Signos de infección podrían ser fiebre o dolor de garganta.
- Alteraciones de la visión, falta de coordinación, torpeza, pérdida de memoria, dificultad para hablar o entender lo que otros dicen, y debilidad en los músculos. Estos podrían ser signos de una infección en el cerebro denominada leucoencefalopatía multifocal progresiva.
- Problemas en el cerebro con signos como convulsiones, confusión, sensación de desorientación, menor sensibilidad, cambios de personalidad, agitación, falta de sueño, alteraciones de la visión, ceguera, coma, parálisis de parte o todo el cuerpo, tortícolis, falta de coordinación con o sin habla anormal o movimiento de los ojos.
- Inflamación en el fondo del ojo que puede estar asociada con visión borrosa. Puede asimismo afectar su visión debido a un aumento en la presión dentro de la cabeza (hipertensión intracraneal benigna).
- Lesión y alteración en el hígado con o sin coloración amarilla en los ojos o piel, náuseas, pérdida de visión y orina oscura.
- Problemas en los riñones, que pueden disminuir en gran medida la cantidad de orina que produce.
- Disminución del número de glóbulos rojos o plaquetas. Estos signos incluyen piel pálida, cansancio, falta de aliento, orina oscura (signo de rotura de los glóbulos rojos), sangrado o hematomas sin razones aparentes, confusión, desorientación, falta de atención y problemas en los riñones.
Otros efectos adversos incluyen:
Muy frecuentes:pueden afectar a más de 1 de cada 10personas
- Problemas en los riñones.
- Presión sanguínea alta.
- Dolor de cabeza.
- Agitación del cuerpo que no se puede controlar.
- Crecimiento excesivo del vello de la cara y del cuerpo.
- Aumento de lípidos en sangre.
Si alguno de ellos le afecta gravemente, informe a su médico.
Frecuentes:pueden afectar hasta 1 de cada 10personas
- Ataques (convulsiones).
- Problemas en el hígado.
- Aumento de azúcar en sangre.
- Cansancio.
- Pérdida de apetito.
- Náuseas (sensación de mareo), vómitos, malestar/dolor abdominal, diarrea.
- Crecimiento excesivo del vello
- Acné, sofocos.
- Fiebre.
- Disminución del número de glóbulos blancos.
- Sensación de entumecimiento u hormigueo.
- Dolor en los músculos, espasmos musculares.
- Úlcera de estómago.
- Crecimiento excesivo del tejido de las encías, que pueden cubrir sus dientes.
- Exceso de ácido úrico o potasio en la sangre, disminución de los niveles de magnesio en la sangre.
Si alguno de ellos le afecta gravemente, informe a su médico.
Poco frecuentes:pueden afectar hasta 1 de cada 100personas
- Síntomas de alteraciones en el cerebro incluyendo ataques repentinos, confusión mental, falta de sueño, desorientación, alteración de la visión, inconsciencia, sensación de debilidad en las extremidades, deterioro del movimiento.
- Erupción en la piel.
- Inflamación generalizada.
- Aumento de peso.
- Disminución del número de glóbulos rojos y plaquetas en sangre que pueden dar lugar a un mayor riesgo de sangrado.
Si alguno de ellos le afecta gravemente, informe a su médico.
Raros: pueden afectar hasta 1 de cada 1.000personas
- Alteración de los nervios con entumecimiento u hormigueo en los dedos de manos y pies.
- Inflamación del páncreas con dolor grave de la parte superior del estómago.
- Debilidad en los músculos, pérdida de fuerza en los músculos, dolor en los músculos de las piernas o las manos o cualquier parte del cuerpo.
- Destrucción de glóbulos rojos, que incluye problemas en los riñones con síntomas como hinchazón de la cara, estómago, manos y/o pies, disminución de la orina, dificultad para respirar, dolor de pecho, ataques, inconsciencia.
- Cambios en el ciclo menstrual, aumento de las mamas en el hombre.
Si alguno de ellos le afecta gravemente, informe a su médico.
Muy raros:pueden afectar hasta 1 de cada 10.000personas
- Inflamación en el fondo del ojo que puede estar asociada con un aumento en la presión dentro de la cabeza y deterioro de la vista.
Si esto le afecta gravemente, informe a su médico.
Frecuencia no conocida:No puede estimarse a partir de los datos disponibles.
- Problemas en el hígado graves con y sin coloración amarilla en los ojos o piel, náuseas (sensación de mareo), pérdida de apetito, coloración oscura de la orina, inflamación de la cara, pies, manos y/o cuerpo entero.
- Sangrado por debajo de la piel o manchas en la piel de color púrpura, sangrado repentino sin causa aparente.
- Migraña o dolor de cabeza grave, a menudo con mareo o sensación del mismo (náuseas, vómitos) y sensibilidad a la luz.
- Dolor en las piernas y los pies.
- Deficiencia auditiva
Si alguno de ellos le afecta gravemente, informe a su médico.
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Otros efectos adversos en niños y adolescentes
No se esperan efectos adversos adicionales en niños y adolescentes comparado con adultos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano (www.notificaRAM.es). Mediante la comunicación de efectos adversos, usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Sandimmun Neoral
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase.
- Conservar a temperatura ambiente (de 15°C a 30°C).
- No refrigerar. No conservar por debajo de 20°C durante más de 1 mes. Esto es debido a que este producto contiene aceites que pueden volverse sólidos a bajas temperaturas.
- Si el medicamento se coloca en la nevera por error, déjelo a temperatura ambiente antes de utilizarlo de nuevo. Copos o pequeñas partículas (sedimentos) en el medicamento no afectan al funcionamiento del mismo o a la seguridad de su uso. La dosis aún puede medirse correctamente con la jeringa.
- El contenido del frasco es estable durante 2 meses después de abierto. Transcurridos 2 meses, debe usar un nuevo frasco.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGREde la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Sandimmun Neoral
- El principio activo es ciclosporina. Un ml de solución oral contiene 100 mg de ciclosporina.
- Los demás componentes son: DL-alfa-tocoferol, etanol anhidro, propilenglicol, mono-di-triglicéridos de aceite de maíz, hidroxiestearato de macrogolglicerol (F.Eur.)/aceite de ricino polioxilo hidrogenado (USP).
Aspecto de Sandimmun Neoral y contenido del envase
Sandimmun Neoral se presenta en forma de una solución oral. Es un líquido trasparente, de color ligeramente amarillento a amarronado.
Está disponible en envases de 20 ml y 50 ml de solución oral y en envases de 50 ml de solución oral con 1 equipo de dispensación oral (jeringa) y 50 ml de solución oral con 2 equipos de dispensación oral (jeringas).
- La jeringa de 1 ml se utiliza para medir volúmenes menores o iguales a 1 ml. Cada graduación de 0,05 ml corresponde a 5 mg de ciclosporina.
- La jeringa de 4 ml se utiliza para medir volúmenes desde 1 ml hasta 4 ml. Cada graduación de 0,1 ml corresponde a 10 mg de ciclosporina.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 – Barcelona (España)
Teléfono: +34 93 306 42 00
Responsable de la fabricación
Jakov-Lind-Straße 5, Top 3.05 1020 Wien Austria
Medialaan 40/Bus 1 1800 Vilvoorde Bélgica
Roonstrasse 25 90429 Nürnberg Alemania
179 Giannou Kranidioti 2235 Latsia, Nicosia Chipre
Edvard Thomsens Vej 14 2300 Copenhagen S Dinamarca
Metsänneidonkuja 10 02130 Espoo Finlandia
8-10, rue Henri Sainte-Claire Deville 92500 Rueil-Malmaison Francia
12th km National Road Athens-Lamia 14451 Metamorphoses Grecia
Bartók Béla út 43-47. 1114 Budapest Hungría |
Via Provinciale Schito 131 80058 Torre Annunziata, NA Italia
Avenida Professor Doutor Cavaco Silva, n.º 10E Taguspark 2740-255 Porto Salvo Portugal
Gran Via de les Corts Catalanes, 764 08013 Barcelona España
Torshamnsgatan 48 164 40 Kista Suecia
Haaksbergweg 16 1101 BX Amsterdam Holanda
Viale Luigi Sturzo, 43 20154-Milano (MI) Italia
15 Marynarska Street 02-674 Warsaw Polonia |
Sophie-Germain-Strasse 10 90443 Nürnberg Alemania |
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo y en el Reino Unido (Irlanda del Norte) con los siguientes nombres:
Alemania | Sandimmun Optoral |
Austria, Bulgaria, Croacia, Chipre, República Checa, Dinamarca, Grecia, Finlandia, Hungría, Islandia, Italia, Malta, Noruega, Polonia, Portugal, Rumania, Eslovaquia, Eslovenia, Suecia, España | Sandimmun Neoral |
Bélgica, Luxemburgo | Neoral-Sandimmun |
Irlanda, Países Bajos, Reino Unido (Irlanda del Norte) | Neoral |
Francia | Néoral |
Fecha de la última revisión de este prospecto: 05/2023
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
Jeringa ??0123. El dispositivo se halla en conformidad con la Directiva 93/42/CEE
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SANDIMMUN NEORAL 100 mg/ml SOLUCION ORALForma farmacéutica: CAPSULA, 100 mgPrincipio activo: ciclosporinFabricante: Teva Pharma S.L.U.Requiere recetaForma farmacéutica: CAPSULA, 25 mgPrincipio activo: ciclosporinFabricante: Teva Pharma S.L.U.Requiere recetaForma farmacéutica: CAPSULA, 50 mgPrincipio activo: ciclosporinFabricante: Teva Pharma S.L.U.Requiere receta
Médicos online para SANDIMMUN NEORAL 100 mg/ml SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SANDIMMUN NEORAL 100 mg/ml SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes