
NUMETA G16%E, EMULSION PARA PERFUSION

Cómo usar NUMETA G16%E, EMULSION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
NUMETA G16%E emulsión para perfusión
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte al médico, farmacéutico o enfermero de su hijo.
- Si su hijo experimenta efectos adversos consulte al médico, farmacéutico o enfermero de su hijo, incluso si se trata de efectos adversos que no aparecen en este prospecto.Ver sección 4.
Contenido del prospecto:
- Qué es Numeta G16%E y para qué se utiliza
- Qué necesita saber antes que Numeta G16%E le sea administrado a su hijo
- Cómo le administrarán Numeta G16%E
- Posibles efectos adversos
- Conservación de Numeta G16%E
- Contenido del envase e información adicional
1. Qué es Numeta G16%E y para qué se utiliza
Numeta G16%E es una nutrición especializada diseñada para los niños recién nacidos a término y hasta los 2 años. Se administra a través de un tubo conectado a la vena de su hijo, cuando no es capaz de comer todos sus alimentos por la boca
Numeta se presenta en forma de una bolsa tricompartimental con tres cámaras independientes que contienen:
una solución de glucosa al 50%
una solución pediátrica de aminoácidos al 5,9% con electrolitos
una emulsión de lípidos (grasas) al 12,5%
Dependiendo de las necesidades de su hijo, dos (o tres de estas soluciones) se mezclan en la bolsa antes de que se le administre a su hijo.
Numeta G16%E sólo debe utilizarse bajo supervisión médica.
2. Qué necesita saber antes que Numeta G16%E le sea administrado a su hijo
No useNumetaG16%E en los siguientes casos:
Con 2 soluciones mezcladas en la bolsa (“2 en1”):
- Si su hijo es alérgico a cualquiera de las proteinas del huevo, de la soja o de los cacahuetes o a los ingrediente de la bolsa de glucosa o de aminoácidos (incluidos en la sección 6).
- Si el organismo de su hijo tiene problemas utilizando los constituyentes de las proteínas.
- Si su hijo tiene en su sangre concentraciones altas de cualquiera de los electrolitos incluidos en Numeta G16%E.
- Si su hijo es un recién nacido (≤ 28 días de edad) Numeta G16%E (u otras soluciones que contienen calcio) no se le debe administrar ceftriaxona (un antibiótico) al mismo tiempo incluso si se usan líneas para la perfusión por separado. Hay un riesgo de formación de partículas en la sangre del recién nacido que puede ser mortal)
- Si su hijo tiene hiperglucemia (niveles especialmente altos de azúcar en la sangre).
Con 3 soluciones mezcladas en la bolsa (“3 en1”).
- Todas las situaciones mencionadas en “2 en 1” más la siguiente:
- Si su hijo tiene un nivel particularmente elevado de grasas en su sangre.
En todos los casos, su médico decidirá si se le debe administrar este medicamento a su hijo en función de factores como la edad, peso y estado clínico, junto con los resultados de todas las pruebas realizadas.
Advertencias y precauciones
Consulte al médico o enfermero de su hijo antes de que le administren Numeta G16%E.
Cuando se utilice en recién nacidos y niños menores de 2 años, la emulsión (en las bolsas y equipos de administración) se debe proteger de la exposición a la luz hasta que finalice la administración. La exposición de Numeta G16%E a la luz ambiental, en especial después de mezclarlo con oligoelementos o vitaminas, genera peróxidos y otros productos de degradación que pueden reducirse si se protege el producto de la exposición a la luz.
Reacciones alérgicas
La perfusión debe detenerse inmediatamente si aparece cualquier signo o síntoma de reacción alérgica (como fiebre, sudoración, escalofríos, cefalea, erupciones cutáneas o dificultad respiratoria). Este medicamento contiene aceite de soja, que raramente puede producir reacciones de hipersensibilidad. En raros casos se ha observado que algunas personas que son alérgicas a las proteínas del cacahuete también lo son a las proteínas de la semilla de soja.
Numeta G16%E contiene glucosa procedente de almidón de maíz, por lo que debe usarse con precaución en pacientes con alergia conocida al maíz o a sus productos.
Riesgo de formación de partículas con la ceftriaxona (antibiótico):
No se debe mezclar ni administrar un antibiótico específico llamado ceftriaxona al mismo tiempo que una solución que contenga calcio (incluida Numeta G16%E) a través de un goteo en su vena.
Su médico conoce esto y no los administrará conjuntamente, ni siquiera a través de diferentes líneas o diferentes sitios de perfusión.
Sin embargo, su médico puede administrar calcio y ceftriaxona secuencialmente uno tras otro si se utilizan líneas de infusión en diferentes puntos, o si se sustituyen las líneas de infusión o si son enjuagadas a fondo con solución salina fisiológica para evitar la formación de precipitados.
Formación de pequeñas partículas en los vasos sanguíneos de los pulmones:
La dificultad respiratoria también puede ser una señal de la formación de pequeñas partículas, que obstruyen los vasos sanguíneos de los pulmones (precipitados vasculares pulmonares). Si su hijo nota dificultad respiratoria, informe al médico o enfermero de su hijo. Ellos decidirán las medidas que deben adoptarse.
Infección y sepsis
Su médico observará atentamente a su hijo para detectar cualquier síntoma de infección. La aplicación de una “técnica aséptica” (técnica sin gérmenes) al colocar y mantener el catéter, así como al preparar la fórmula de nutrición, puede reducir el riesgo de infección.
En ocasiones, los niños pueden desarrollar infecciones y sepsis (bacterias en la sangre) cuando tienen un tubo conectado a la vena (catéter intravenoso). Ciertos medicamentos y enfermedades pueden aumentar el riesgo de desarrollar sepsis o infección. Los pacientes que requieren nutrición parenteral (nutrición administrada a través de un tubo conectado a la vena de su hijo) tienen más posibilidad de desarrollar una infección debido a su estado clínico.
Síndrome de sobrecarga de grasa
Se han descrito casos de síndrome de sobrecarga de grasa con productos similares. Una reducción de la capacidad de eliminación de los lípidos que contiene Numeta G16%E, o una sobredosis, puede ocasionar un "síndrome de sobrecarga de grasa" (ver secciones 3 y 4 Posibles efectos adversos).
Cambio en los niveles de sustancias químicas de la sangre
Su médico comprobará y revisará los líquidos en el organismo, los niveles de sustancias químicas en la sangre y otros niveles en la sangre de su hijo ya que en ocasiones, la realimentación a alguien que está gravemente desnutrido puede dar lugar a cambios en los niveles de química sanguínea. También se puede producir un exceso de fluido en los tejidos e hinchazón. Es recomendable iniciar la nutrición parenteral lentamente y bajo supervisión.
Niveles elevados de magnesio en sangre
La cantidad de magnesio presente en Numeta G16%E puede dar lugar niveles elevados de magnesio en la sangre. Entre los signos de estos niveles elevados se incluyen debilidad, lentitud de reflejos, náuseas, vómitos, niveles bajos de calcio en sangre, dificultades de la respiración, presión arterial baja y latidos irregulares del corazón Dado que es posible que sea difícil detectar estos signos, el médico puede controlar los valores en sangre de su hijo, especialmente si su hijo tiene factores de riesgo relacionados con niveles elevados de magnesio en sangre, lo que incluye una función renal alterada. Si los niveles de magnesio en sangre son elevados, se detendrá o reducirá la perfusión.
Supervisión y ajuste
Su médico supervisará y adaptará la dosis de Numeta G16%E para que se ajuste a las necesidades individuales de su hijo si presenta los siguientes estados:
- estado postraumático grave
- diabetes mellitus grave
- shock
- infarto
- infección grave
- ciertos tipos de coma
Uso con precaución:
Numeta G16%E debe ser utilizado con precaución si su hijo tiene:
- edema pulmonar (líquido en los pulmones) o insuficiencia cardiaca,
- problemas hepáticos graves,
- problemas para asimilar nutrientes,
- altos niveles de azúcar,
- problemas renales,
- alteraciones metabólicas graves (cuando su organismo no puede eliminar las sustancias de forma normal),
- alteraciones de la coagulación de la sangre.
Los niveles de líquidos en el organismo de su hijo, los valores de la analítica del hígado y/o los valores de la sangre serán cuidadosamente controlados.
Uso de Numeta G16%E con otros medicamentos
Informe a su médico si su hijo está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Numeta G16%Eno debe administrarse al mismo tiempo que:
- ceftriaxona(un antibiótico) ni siquiera en vías de perfusión separadas, debido al riesgo de formación de partículas.
- sangrea través de la misma vía de perfusión. Debido al riesgo de pseudoaglutinación (los glóbulos rojos quedan apilados)
- ampicilina,fosfenitoina y furosemidaa través de la misma vía de perfusión,ya que existe el riesgo de formación de partículas.
Cumarina y warfarina (anticoagulantes)
El médico observará atentamente a su hijo si éste está tomando cumarina o warfarina. El aceite de oliva y de soja contienen vitamina K1. La vitamina K1 puede interferir en la acción de medicamentos como la cumarina y la warfarina. Estos medicamentos son anticoagulantes que se utilizan para evitar la coagulación de sangre.
Pruebas de laboratorio
Los lípidos incluidos en esta emulsión pueden interferir con los resultados de ciertas pruebas de laboratorio. Las pruebas de laboratorio se pueden realizar tras un período de 5 a 6 horas si no se administran más lípidos.
Interacción de Numeta G16%Econ medicamentos que puedan afectar a los niveles/metabolismo del potasio:
Numeta G16%E contiene potasio. Los niveles altos de potasio en sangre pueden causar un ritmo cardíaco anormal. Se debe prestar especial atención a los pacientes que tomen diuréticos (medicamentos que reducen la retención de fluidos), inhibidores de la ECA o antagonistas del receptor II de la angiotensina (todos ellos medicamentos utilizados para el tratamiento de la presión arterial elevada) o con inmunosupresores (medicamentos que pueden disminuir las defensas naturales del organismo). Este tipo de medicamentos puede aumentar los niveles de potasio.
3. Cómo le administrarán Numeta G16%E
A su hijo siempre le deben administrar Numeta G16%E exactamente como lo haya indicado su médico. Consulte a su médico si tiene dudas.
Rango de edad
Numeta G16%E se ha diseñado para que se adapte a las necesidades nutricionales de niños nacidos a término y niños hasta dos años.
El médico decidirá si este medicamento es adecuado para su hijo.
Administración
Este medicamento es una emulsión para perfusión. Se administra a través de un tubo de plástico conectado a una vena del brazo o a una vena grande del pecho de su hijo.
El médico de su hijo puede optar por no administrar lípidos a su hijo. El diseño de la bolsa de Numeta G16%E permite romper solo el sello no permanente del compartimento entre las cámaras de los aminoácidos/electrolitos y glucosa, si es necesario. El sello entre las camáras de aminoácidos y lípidos se mantiene intacto en este caso. El contenido de la bolsa puede ser perfundido sin lípidos.
Cuando se utilice en recién nacidos y niños menores de 2 años, la emulsión (en las bolsas y equipos de administración) se debe proteger de la exposición a la luz hasta que finalice la administración (ver sección 2).
Dosificación y duración del tratamiento
El médico de su hijo decidirá la dosis que necesitará el niño y cuanto tiempo se le administrará. La dosis depende de las necesidades nutricionales de su hijo y se basará en el peso, el estado médico y la capacidad del cuerpo de su hijo para digerir y absorber los ingredientes de Numeta G16%E. También podrían administrarse proteínas o nutrición adicional de forma oral o intestinal.
Si a su hijo le administran másNumetaG16%Edel que debe
En caso de sobredosis o ingestión accidental, consultar al Servicio de Información Toxicológica. Teléfono 915.620.420
Síntomas
Recibir demasiado medicamento o hacerlo muy rápidamente podría provocar:
- náuseas (sentirse indispuesto)
- vómitos
- escalofríos
- alteraciones electrolíticas (cantidades inapropiadas de electrolitos en sangre)
- signos de hipervolemia (aumento del volumen de circulación sanguínea)
- acidosis (aumento de la acidez de la sangre)
En esos casos, debe detenerse la perfusión inmediatamente. El médico de su hijo decidirá si es necesario realizar otras acciones.
Una sobredosis de las grasas contenidas en Numeta G16%E puede provocar un "síndrome de sobrecarga de grasa", que suele ser reversible una vez interrumpida la infusión. En recién nacidos (neonatos) y niños pequeños (bebés), el síndrome de sobrecarga de grasa se ha asociado con trastornos respiratorios que provocan una reducción del oxígeno en el cuerpo (dificultad respiratoria) y afecciones que provocan un aumento de la acidez de la sangre (acidosis).
Para evitar que se produzcan estas reacciones, su médico supervisará regularmente el estado de su hijo y analizará sus niveles sanguíneos durante el tratamiento.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todos los niños los sufran.
Si observa algún cambio en la forma en que se siente su hijo durante el tratamiento o después de él, comuníquelo inmediatamente al médico o enfermero.
Las pruebas que el médico le realizará a su hijo mientras recibe este medicamento deben minimizar el riesgo de efectos adversos.
Si se presentan síntomas de una reacción alérgica, la perfusión deberá detenerse y se contactará con el médico inmediatamente. Esto puede ser grave y los síntomas pueden incluir:
- Sudoración.
- Escalofríos.
- Dolor de cabeza.
- Erupciones cutáneas.
- Dificultad para respirar.
Otros efectos adversos que se han observado son:
Frecuentes: pueden afectar hasta 1 de cada 10 personas
- Bajo nivel de fosfato en sangre (hipofosfatemia).
- Alto nivel de azúcar en sangre (hiperglucemia).
- Alto nivel de calcio en sangre (hipercalcemia).
- Alto nivel de triglicéridos en sangre (hipertrigliceridemia).
- Alteraciones electrolíticas (hiponatremia).
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
- Alto nivel de lípidos en sangre (hiperlipidemia).
- Un trastorno en el que la bilis no puede fluir desde el hígado hasta el duodeno (colestasis). El duodeno es parte del intestino.
Desconocidos: la frecuencia no puede estimarse a partir de los datos disponibles (estos efectos adversos se han comunicado con Numeta G13%E y G16%E cuando se han administrado por vía periférica con una dilución insuficiente).
- Necrosis de la piel
- Daño en tejido blando
- Extravasación
Se han notificado los siguientes efectos adversos con otros productos para nutrición parenteral:
- La capacidad reducida o limitada para eliminar los lípidos que contiene Numeta puede dar lugar a un “síndrome de sobrecarga de grasa”. Los siguientes signos y síntomas de este síndrome suelen ser reversibles cuando se detiene la perfusión de la emulsión de lípidos:
- Empeoramiento repentino y brusco del estado médico del paciente.
- Alto nivel de grasa en sangre (hiperlipidemia).
- Fiebre.
- Infiltración grasa del hígado (hepatomegalia).
- Empeoramiento de la función hepática.
- Disminución de los glóbulos rojos, lo que puede hacer palidecer la piel y producir debilidad o dificultad para respirar (anemia).
- Bajo recuento de glóbulos blancos, lo que puede incrementar el riesgo de infección (leucocitopenia).
- Bajo recuento de plaquetas, lo que puede incrementar el riesgo de hematomas y/o hemorragias (trombocitopenia).
- Trastornos de la coagulación, lo que afecta a la capacidad de la sangre para coagularse
- Trastorno respiratorio que produce una reducción del oxígeno en el cuerpo (dificultad respiratoria)
- Condiciones que provocan un aumento de la acidez de la sangre (acidosis)
- Estado de coma que requiere hospitalización.
- Formación de pequeñas partículas que pueden provocar la obstrucción de los vasos sanguíneos de los pulmones (precipitados vasculares pulmonares) o dificultad para respirar.
Comunicación de efectos adversos:
Si su hijo experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Numeta G16%E
Mantener este medicamento fuera de la vista y del alcance de los niños cuando no se está administrando.
Cuando se utilice en recién nacidos y niños menores de 2 años, la emulsión (en las bolsas y equipos de administración) se debe proteger de la exposición a la luz hasta que finalice la administración (ver sección 2).
No utilice este medicamento después de la fecha de caducidad que aparece en la bolsa y el embalaje exterior (MM/AAAA). La fecha de caducidad es el último día del mes que se indica.
No congelar.
Conservar en la sobrebolsa.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Aspecto del producto y contenido del envase
Numeta G16%E se presenta en forma de bolsa con tres cámaras. Cada bolsa contiene una combinación estéril de una solución de glucosa, una solución pediátrica de aminoácidos con electrolitos y una emulsión de lípidos, tal como se describe a continuación:
Tamaño del envase | Solución deglucosa al 50% | Solución de aminoácidos al 5,9% con electrolitos | Emulsión delípidos al 12,5% |
500 ml | 155 ml | 221 ml | 124 ml |
Aspecto antes de la reconstitución:
- Las soluciones de las cámaras de aminoácidos y glucosa son transparentes, incoloras o ligeramente amarillentas.
- La cámara de la emulsión de lípidos tiene un aspecto lechoso y blanquecino.
Aspecto después de la reconstitución:
- La solución “2 en 1” es transparente, incolora o ligeramente amarillenta.
- La emulsión para perfusión “3 en 1” tiene un aspecto uniforme lechoso y blanquecino.
La bolsa con tres compartimientos es una bolsa de plástico multicapa.
Para evitar el contacto con el aire, Numeta está envasada en el interior de una sobrebolsa de barrera de oxígeno que contiene un sobrecito con un absorbente de oxígeno y un indicador de oxígeno.
Tamaños de envase
Bolsa de 500 ml: 6 unidades por caja
1 bolsa de 500 ml
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Baxter, S.L.
Pouet de Camilo 2, 46394 Ribarroja del Turia (Valencia)
Responsable de la fabricación
Baxter S.A.
Boulevard Rene Branquart, 80
7860 Lessines
Bélgica
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria Alemania | Numeta G16 % E Emulsion zur Infusion |
Bélgica Luxemburgo | Numetzah G16%E, émulsion pour perfusion |
Francia | Numetah G16 %E émulsion pour perfusion |
Dinamarca Noruega Suecia | Numeta G16E |
República Checa Grecia | Numeta G16 % E |
Holanda | Numeta G16%E emulsie voor infusie |
Irlanda Reino Unido | Numeta G16%E, Emulsion for Infusion |
Italia | Numeta G16%E emulsione per infusione |
Finlandia | Numeta G16E infuusioneste, emulsio |
Polonia | Numeta G16 % E |
Portugal | Numeta G16%E |
España | Numeta G16%E, emulsión para perfusión |
Fecha de la última revisión de este prospecto: Mayo 2024
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española del Medicamento y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
---------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:*
(*) Tenga en cuenta que en ciertos casos estos medicamentos pueden ser administrados en casa por los padres u otros cuidadores. En estos casos, los padres/cuidadores deben leer la siguiente información.
No debe añadirse ningún medicamento a la bolsa sin comprobar primero la compatibilidad. Podrían formarse partículas o que se descomponga la emulsión de lípidos, lo que podría bloquear los vasos sanguíneos.
Numeta G16%E debe estar a temperatura ambiente antes de su uso.
Antes de administrar Numeta G16%E, deberá preparar la bolsa como se muestra a continuación.
Asegúrese de que la bolsa no esté dañada y utilícela solamente si no presenta daños. Una bolsa no dañada tiene este aspecto:
- Los sellos no permanentes están intactos. Esto se observa porque no existe una mezcla de las tres cámaras.
- La solución de aminoácidos y de glucosa es transparente, incolora o ligeramente amarillenta y sin partículas visibles.
- La emulsión de lípidos es un líquido de aspecto lechoso y blanquecino.
Antes de abrir la sobrebolsa, examine el color del absorbente de oxígeno.
- Compárelo con el color de referencia impreso junto al símbolo de OK y mostrado en el área impresa de la etiqueta del indicador.
- No utilice el producto si el color del absorbente de oxígeno no corresponde al color de referencia impreso junto al símbolo OK.
Las figuras 1 y 2 ilustran cómo quitar la sobrebolsa. Deséchela junto con el indicador de oxígeno y el absorbente de oxígeno.
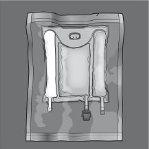
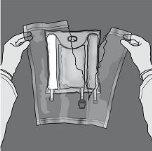
Figura 1 Figura 2
Preparación de la mezcla
- Asegúrese de que el producto está a temperatura ambiente antes de romper los sellos no permanentes.
- Coloque la bolsa en una superficie limpia y plana.
Activación de la bolsa de 3 cámaras (rotura de los dos sellos no permanentes)
Paso 1: Enrolle la bolsa desde el lado del colgador en D
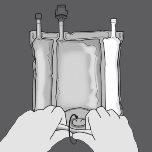
Paso 2: Presione hasta que se abran los sellos no permanentes.
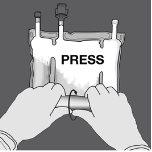
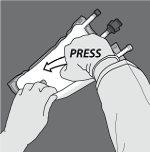
Paso 3: Cambie el sentido y enrolle la bolsa hacia el colgador en D hasta que el sello esté completamente abierto. Siga los mismos pasos para abrir el segundo sello no permanente.
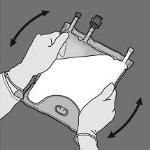
Paso 4: De la vuelta a la bolsa al menos tres veces para mezclar bien el contenido. El aspecto de la solución mezclada debe ser una emulsión de color blanco lechoso.
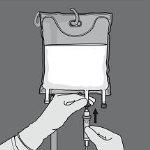
Paso 5: Retire el tapón protector del punto de administración e inserte el equipo de administración intravenoso.
Activación de la bolsa de 2 cámaras (rotura del sello no permanente situado entre las cámaras de aminoácidos y glucosa)
Paso 1: Para romper solo el sello no permanente de aminoácidos/glucosa, comience a enrollar la bolsa desde la esquina del colgador en D del sello que separa las cámaras de aminoácidos y glucosa y presione para abrir el sello que separa ambos compartimentos.
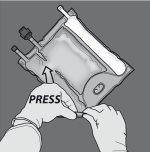
Paso 2: Coloque la bolsa de forma que el compartimento con emulsión de lípidos esté mirando hacia el operador y enrolle la bolsa mientras protege el compartimento con emulsión de lípidos en las palmas de las manos.
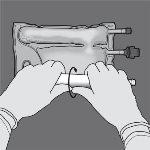
Paso 3: Con una mano, aplique presión enrollando la bolsa hacia los tubos.
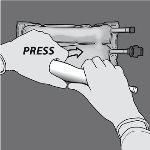
Paso 4: A continuación, cambie el sentido y enrolle la bolsa hacia el colgador en D, presionando con la otra mano hasta que el sello que separa las soluciones de aminoácidos y glucosa se abra completamente.
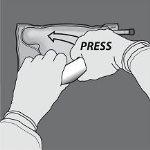
Paso 5: De la vuelta a la bolsa al menos tres veces para mezclar bien el contenido. El aspecto de la solución mezclada debe ser transparente, incoloro o ligeramente amarillento.
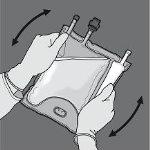
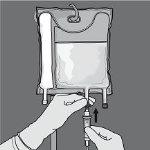
Paso 6: Retire el tapón protector del punto de administración e inserte el equipo de administración intravenoso.
La velocidad de administración debe aumentarse gradualmente durante la primera hora y ajustarse en función de los siguientes factores:
La dosis que se va a administrar
La ingesta del volumen diaria
La duración de la perfusión.
Forma de administración
Cuando se utilice en recien nacidos y niños menores de 2 años, la emulsión (en la bolsa y en el equipo de administración) se debe proteger de la exposición a la luz hasta que finalice la administración.
Se recomienda el uso de un filtro de 1,2 micras para la administración de Numeta G16%E.
Debido a su elevada osmolaridad, Numeta G16%E solo puede administrase sin diluir a través de una vena central; no obstante, una dilución apropiada de Numeta G16%E con agua para preparaciones inyectables reduce la osmolaridad y permite la perfusión periférica.
La siguiente fórmula indica el impacto de la dilución sobre la osmolaridad de las bolsas.
Osmolaridad final | = | Volumen de la bolsa x osmolaridad inicial |
Agua añadida + Volumen de la bolsa |
La siguiente tabla muestra ejemplos de osmolaridad para las adiciones de las bolsas de 2 y 3 cámaras activadas tras la adición de agua para preparaciones inyectables:
Aminoácidos y glucosa(B2C activada) | Aminoácidos, glucosa ylípidos (B3C activada) | |
Volumen inicial en la bolsa (ml) | 376 | 500 |
Osmolaridad inicial (mOsm/l aprox) | 1585 | 1230 |
Volumen de agua añadida (ml) | 376 | 500 |
Volumen final tras adición (ml) | 752 | 1000 |
Osmolaridad tras adición (mOsm/l aprox) | 792,5 | 615 |
Adición de medicación
Las mezclas que incluyen oligoelementos y vitaminas deben protegerse de la luz, desde el punto de administración y durante la administración. La exposición a la luz ambiente genera peróxidos y otros productos de degradación los cuales pueden reducirse con fotoprotección
Se pueden añadir medicamentos compatibles en la mezcla reconstituida (después de abrir los sellos no permanentes y mezclar el contenido de las dos o tres cámaras) a través del punto de inyección.
También se pueden añadir vitaminas a la cámara de glucosa antes de reconstituir la mezcla (antes de abrir los sellos no permanentes y mezclar las soluciones y la emulsión).
En las Tablas 1-6 se muestran las posibles adiciones de soluciones de oligoelementos disponibles comercialmente (identificadas como TE1, TE2 y TE4), vitaminas (identificadas como liofilizado V1 y emulsión V2) y electrolitos en cantidades definidas.
1 Compatibilidad con TE4, V1 y V2
Tabla 1: Compatibilidad de 3-en-1 (B3C activadas) con o sin dilución en agua
Por 500 ml (mezcla 3 en 1 con lípidos) | ||||||
Mezcla sin diluir | Mezcla diluida | |||||
Aditivos | Nivel incluido | Adición máxima | Nivelmáximo total | Nivel incluido | Adición máxima | Nivelmáximo total |
Sodio (mmol) | 12,0 | 25,6 | 37,6 | 12,0 | 25,6 | 37,6 |
Potasio (mmol) | 11,4 | 26,2 | 37,6 | 11,4 | 26,2 | 37,6 |
Magnesio (mmol) | 1,6 | 3,6 | 5,2 | 1,6 | 3,6 | 5,2 |
Calcio (mmol) | 3,1 | 16,4 | 19,5 | 3,1 | 8,2 | 11,3 |
Fosfato* (mmol) | 4,4 | 6,9 | 11,3 | 4,4 | 6,9 | 11,3 |
Oligoelementos y vitaminas | - | 10 ml TE4 +1 vial V1 +30 ml V2 | 10 ml TE4 +1 vial V1 +30 ml V2 | - | 5 ml TE4+ ½ vial V1 +5 ml V2 | 5 ml TE4+ ½ vial V1 +5 ml V2 |
Agua para preparaciones inyectables | - | - | - | - | 350 ml | 350 ml |
- Fosfato orgánico
Tabla 2: Compatibilidad de 2-en-1 (B2C activadas) con o sin dilución en agua
Por 376 ml (mezcla 2 en 1 sin lípidos)) | ||||||
Mezcla sin diluir | Mezcla diluida | |||||
Aditivos | Nivel incluido | Adición máxima | Nivelmáximo total | Nivel incluido | Adición máxima | Nivelmáximo total |
Sodio (mmol) | 11,6 | 26,0 | 37,6 | 11,6 | 0,0 | 11,6 |
Potasio (mmol) | 11,4 | 26,2 | 37,6 | 11,4 | 0,0 | 11,4 |
Magnesio (mmol) | 1,6 | 3,6 | 5,2 | 1,6 | 0,0 | 1,6 |
Calcio (mmol) | 3,1 | 8,2 | 11,3 | 3,1 | 0,0 | 3,1 |
Fosfato* (mmol) | 3,2 | 8,1 | 11,3 | 3,2 | 0,0 | 3,2 |
Oligoelementos y vitaminas | - | 5ml TE4+ ½ vial V1 | 5ml TE4+ ½ vial V1 | - | 5ml TE4+ ½ vial V1 | 5ml TE4+ ½ vial V1 |
Agua para preparaciones inyectables | - | - | - | - | 450 ml | 450 ml |
- Fosfato orgánico
2 Compatibilidad con TE1, V1 y V2
Tabla 3: Compatibilidad de 3-en-1 (B3C activadas) con o sin dilución en agua
Por 500 ml (mezcla 3 en 1 con lípidos) | ||||||
Mezcla sin diluir | Mezcla diluida | |||||
Aditivos | Nivel incluido | Adiciónmáxima | Nivelmáximo total | Nivel incluido | Adición máxima | Nivelmáximo total |
Sodio (mmol) | 12,0 | 4,0 | 16,0 | 12,0 | 0,0 | 12,0 |
Potasio (mmol) | 11,4 | 6,2 | 17,6 | 11,4 | 0,0 | 11,4 |
Magnesio (mmol) | 1,6 | 0 | 1,6 | 1,6 | 0,0 | 1,6 |
Calcio (mmol) | 3,1 | 2,1 | 5,2 | 3,1 | 0,0 | 3,1 |
Fosfato* (mmol) | 4,4 | 2,0 | 6,4 | 4,4 | 0,0 | 4,4 |
Oligoelementos y vitaminas | - | 5 ml TE1 +½ vial V1 + 5 ml V2 | 5 ml TE1 +½ vial V1 + 5 ml V2 | - | 5 ml TE1 +½ vial V1 + 5 ml V2 | 5 ml TE1 +½ vial V1 + 5 ml V2 |
Agua para preparaciones inyectables | - | - | - | - | 350 ml | 350 ml |
- Fosfato orgánico
Tabla 4: Compatibilidad de 2-en-1 (B2C activadas) con o sin dilución en agua
Por 376 ml (mezcla 2 en 1 sin lípidos)) | ||||||
Mezcla sin diluir | Mezcla diluida | |||||
Aditivos | Nivel incluido | Adición máxima | Nivelmáximo total | Nivel incluido | Adición máxima | Nivelmáximo total |
Sodio (mmol) | 11,6 | 26,0 | 37,6 | 11,6 | 0,0 | 11,6 |
Potasio (mmol) | 11,4 | 26,2 | 37,6 | 11,4 | 0,0 | 11,4 |
Magnesio (mmol) | 1,6 | 3,6 | 5,2 | 1,6 | 0,0 | 1,6 |
Calcio (mmol) | 3,1 | 8,2 | 11,3 | 3,1 | 0,0 | 3,1 |
Fosfato* (mmol) | 3,2 | 8,1 | 11,3 | 3,2 | 0,0 | 3,2 |
Oligoelementos y vitaminas | - | 5 ml TE1+ ½ vial V1 | 5 ml TE1+ ½ vial V1 | - | 5 ml TE1+ ½ vial V1 | 5 ml TE1+ ½ vial V1 |
Agua para preparaciones inyectables | - | - | - | - | 450 ml | 450 ml |
- Fosfato orgánico
3 Compatibilidad con TE2, V1 y V2
Tabla 5: Compatibilidad de 3-en-1 (B3C activadas) con o sin dilución en agua
Por 500 ml (mezcla 3 en 1 con lípidos) | ||||||
Mezcla sin diluir | Mezcla diluida | |||||
Aditivos | Nivel incluido | Adiciónmáxima | Nivelmáximo total | Nivel incluido | Adición máxima | Nivelmáximo total |
Sodio (mmol) | 12,0 | 4,0 | 16,0 | 12,0 | 0,0 | 12,0 |
Potasio (mmol) | 11,4 | 6,2 | 17,6 | 11,4 | 0,0 | 11,4 |
Magnesio (mmol) | 1,6 | 0 | 1,6 | 1,6 | 0,0 | 1,6 |
Calcio (mmol) | 3,1 | 2,1 | 5,2 | 3,1 | 0,0 | 3,1 |
Fosfato* (mmol) | 4,4 | 2,0 | 6,4 | 4,4 | 0,0 | 4,4 |
Oligoelementos y vitaminas | - | 5 ml TE2 +½ vial V1 + 5 ml V2 | 5 ml TE2 +½ vial V1 + 5 ml V2 | - | 5 ml TE2 +½ vial V1 + 5 ml V2 | 5 ml TE2 +½ vial V1 + 5 ml V2 |
Agua para preparaciones inyectables | - | - | - | - | 350 ml | 350 ml |
- Fosfato orgánico
Tabla 6: Compatibilidad de 2-en-1 (B2C activadas) con o sin dilución en agua
Por 376 ml (mezcla 2 en 1 sin lípidos)) | ||||||
Mezcla sin diluir | Mezcla diluida | |||||
Aditivos | Nivel incluido | Adición máxima | Nivelmáximo total | Nivel incluido | Adición máxima | Nivelmáximo total |
Sodio (mmol) | 11,6 | 26,0 | 37,6 | 11,6 | 0,0 | 11,6 |
Potasio (mmol) | 11,4 | 26,2 | 37,6 | 11,4 | 0,0 | 11,4 |
Magnesio (mmol) | 1,6 | 3,6 | 5,2 | 1,6 | 0,0 | 1,6 |
Calcio (mmol) | 3,1 | 8,2 | 11,3 | 3,1 | 0,0 | 3,1 |
Fosfato* (mmol) | 3,2 | 8,1 | 11,3 | 3,2 | 0,0 | 3,2 |
Oligoelementos y vitaminas | - | 5 ml TE2+ ½ vial V1 | 5 ml TE2+ ½ vial V1 | - | 5 ml TE2+ ½ vial V1 | 5 ml TE2+ ½ vial V1 |
Agua para preparaciones inyectables | - | - | - | - | 450 ml | 450 ml |
- Fosfato orgánico
La composición de las preparaciones de vitaminas y oligoelementos se ilustra a continuación en las tablas 7 y 8:
Tabla 7: Composición de la preparación de oligoelementos comerciales utilizada:
Composiciónpor 10 ml | TE1 | TE2 | TE4 |
Hierro | - | 8,9 µmol o 0,5 mg | - |
Zinc | 38,2 µmol o 2,5 mg | 15,3 µmol o 1 mg | 15,3 µmol o 1 mg |
Selenio | 0,253µmol o 0,02 mg | 0,6 µmol o 0,05 mg | 0,253 µmol o 0,02 mg |
Cobre | 3,15 µmol o 0,2 mg | 4,7 µmol o 0,3 mg | 3,15 µmol o 0,2 mg |
Iodo | 0,0788µmol o 0,01 mg | 0,4 µmol o 0,05 mg | 0,079 µmol o 0,01 mg |
Fluor | 30 µmol o 0,57 mg | 26,3 µmol o 0,5 mg | - |
Molibdeno | - | 0,5 µmol o 0,05 mg | - |
Manganeso | 0,182 µmol o 0,01 mg | 1,8 µmol o 0,1 mg | 0,091 µmol o 0,005 mg |
Cobalto | - | 2,5 µmol o 0,15 mg | - |
Cromo | - | 0,4 µmol o 0,02 mg | - |
Tabla 8: Composición de los preparados vitamínicos comerciales utilizados:
Composición por vial | V1 | V2 |
Vitamina B1 | 2,5 mg | - |
Vitamina B2 | 3,6 mg | - |
Nicotinamida | 40 mg | - |
Vitamina B6 | 4,0 mg | - |
Ácido pantoténico | 15,0 mg | - |
Biotina | 60 µg | - |
Ácido fólico | 400 µg | - |
Vitamina B12 | 5,0 µg | - |
Vitamina C | 100 mg | - |
Vitamina A | - | 2300 UI |
Vitamina D | - | 400 UI |
Vitamina E | - | 7 UI |
Vitamina K | - | 200 µg |
Para realizar una adición:
- Se debe llevar a cabo en condiciones asépticas.
- Prepare el punto de inyección de la bolsa.
- Perfore el punto de inyección e inyecte los aditivos utilizando una aguja de inyección o un dispositivo de reconstitución.
- Mezcle el contenido de la bolsa y los aditivos.
Preparación de la perfusión:
- Se debe llevar a cabo en condiciones asépticas.
- Cuelgue la bolsa.
- Retire el protector de plástico de la salida de administración.
- Inserte firmemente la punta del equipo de perfusión en la salida de administración.
Administración de la perfusión:
- Para un solo uso.
- Administre únicamente el producto después de abrir los precintos no permanentes que hay entre las dos o tres cámaras y mezclar el contenido de las dos o tres cámaras.
- Asegúrese de que la emulsión para perfusión de la bolsa de tres cámaras final activada no muestra signos de separación de fases y que la solución para perfusión de la bolsa de dos cámaras final no muestra signos de partículas.
- Se recomienda usar inmediatamente después de la rotura de los sellos no permanentes. No debe almacenarse para una perfusión posterior.
- No conecte una bolsa a medio utilizar.
- No conecte las bolsas en serie para evitar que se produzca una embolia gaseosa a consecuencia del aire residual contenido en la bolsa principal.
- Se recomienda el uso de un filtro de 1,2 micras para la administración de Numeta G16%E.
- Cuando se utilice en recién nacidos y niños menores de 2 años, debe protegerse de la exposición a la luz hasta que finalice la administración. La exposición de Numeta G16%E a la luz ambiental, en especial después de mezclarlo con oligoelementos o vitaminas, genera peróxidos y otros productos de degradación que pueden reducirse si se protege el producto de la exposición a la luz.
- El producto que no se haya utilizado, los materiales que hayan estado en contacto con él y todos los dispositivos desechables necesarios deberán desecharse debidamente.
Período de validez tras mezclar las soluciones
Utilice el producto inmediatamente después de abrir los sellos no permanentes que hay entre las dos o tres cámaras. Se han realizado estudios que demuestran la estabilidad de las mezclas durante 7 días a una temperatura entre 2 °C y 8 °C seguidos de 48 horas a una temperatura que no supere los 30 °C.
Periodo de validez tras la suplementación (electrolitos, oligoelementos, vitaminas, agua):
Para determinadas adiciones, se ha demostrado la estabilidad física de la formulación de Numeta durante 7 días a una temperatura de entre 2 °C y 8 °C seguidos de 48 horas a 30 °C.
Desde el punto de vista microbiológico el producto debe utilizarse inmediatamente. Si no se utiliza inmediatamente, las condiciones y los tiempos de conservación antes de su uso son responsabilidad del usuario y normalmente no superarán las 24 horas de 2 °C a 8 °C, a menos que la reconstitución / dilución / adición se haya llevado a cabo en condiciones asépticas controladas y validadas.
No use Numeta G16%E si la bolsa está dañada. Una bolsa dañada tiene el siguiente aspecto:
- Los sellos no permanentes están rotos.
- Una de las cámaras contiene una mezcla de cualquiera de las soluciones.
- La solución de aminoácidos y de glucosa no es transparente, incolora ni ligeramente amarillenta y/o contiene partículas visibles.
- La emulsión de lípidos no tiene un aspecto lechoso y blanquecino.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
Composición de Numeta G16%E
Los principios activos son:
Composición | 500 ml | |
Principio activo | B2C activada (376 ml) | B3C activada (500 ml) |
Compartimento de aminoácidos | ||
Principio activo | ||
Alanina | 1,03 g | 1,03 g |
Arginina | 1,08 g | 1,08 g |
Ácido aspártico | 0,77 g | 0,77 g |
Cisteína | 0,24 g | 0,24 g |
Ácido glutámico | 1,29 g | 1,29 g |
Glicina | 0,51 g | 0,51 g |
Histidina | 0,49 g | 0,49 g |
Isoleucina | 0,86 g | 0,86 g |
Leucina | 1,29 g | 1,29 g |
Lisina monohidratada(equivalente a Lisina) | 1,59 g (1,42 g) | 1,59 g (1,42 g) |
Metionina | 0,31 g | 0,31 g |
Ornitina HCl(equivalente a Ornitina) | 0,41 g (0,32 g) | 0,41 g (0,32 g) |
Fenilalanina | 0,54 g | 0,54 g |
Prolina | 0,39 g | 0,39 g |
Serina | 0,51 g | 0,51 g |
Taurina | 0,08 g | 0,08 g |
Treonina | 0,48 g | 0,48 g |
Triptófano | 0,26 g | 0,26 g |
Tirosina | 0,10 g | 0,10 g |
Valina | 0,98 g | 0,98 g |
Cloruro de sodio | 0,30 g | 0,30 g |
Acetato de potasio | 1,12 g | 1,12 g |
Cloruro de calcio dihidratado | 0,46 g | 0,46 g |
Acetato magnésico tetrahidratado | 0,33 g | 0,33 g |
Glicerofostato de sodio hidratado | 0,98 g | 0,98 g |
Compartimento de glucosa | ||
Glucosa monohidratada (equivalente a glucosa anhidra) (equivalente a glucosa anhidra) | 85,25 g (77,50 g) | 85,25 g (77,50 g) |
Compartimento de lípidos | ||
Aceite de de soja refinado (aprox. 80%) + aceite de oliva refinado (aprox. 20%) | - | 15,5 g |
La solución/emulsión reconstituida proporciona lo siguiente:
Composición | ||||
B2C activada | B3C activada | |||
Por unidad de volumen (ml) | 376 | 100 | 500 | 100 |
Nitrógeno (g) | 2,0 | 0,52 | 2,0 | 0,39 |
Aminoácidos (g) | 13,0 | 3,5 | 13,0 | 2,6 |
Glucosa (g) | 77,5 | 20,6 | 77,5 | 15,5 |
Lípidos (g) | 0 | 0 | 15,5 | 3,1 |
Energía | ||||
Calorías totales (kcal) | 362 | 96 | 517 | 103 |
Calorías no proteicas (kcal) | 310 | 82 | 465 | 93 |
Calorías de glucosa (kcal) | 310 | 82 | 310 | 62 |
Calorías de lípidos a (kcal) | 0 | 0 | 155 | 31 |
Calorías no proteicas / nitrógeno (kcal/g N) | 158 | 158 | 237 | 237 |
Calorías de lípidos (%calorías no proteicas) | NA | N/A | 33 | 33 |
Calorías de lípidos (%calorías totales) | NA | N/A | 30 | 30 |
Electrolitos | ||||
Sodio (mmol) | 11,6 | 3,1 | 12,0 | 2,4 |
Potasio (mmol) | 11,4 | 3,0 | 11,4 | 2,3 |
Magnesio (mmol) | 1,6 | 0,41 | 1,6 | 0,31 |
Calcio (mmol) | 3,1 | 0,82 | 3,1 | 0,62 |
Fosfatob (mmol) | 3,2 | 0,85 | 4,4 | 0,87 |
Acetato (mmol) | 14,5 | 3,9 | 14,5 | 2,9 |
Malato (mmol) | 4,3 | 1,1 | 4,3 | 0,86 |
Cloruro (mmol) | 13,8 | 3,7 | 13,8 | 2,8 |
pH (aprox.) | 5,5 | 5,5 | 5,5 | 5,5 |
Osmolaridad aprox. (mOsm/L) | 1585 | 1585 | 1230 | 1230 |
(a) Incluye calorías de fosfolípidos de huevo para perfusión.
(b) Incluye el fosfato proporcionado por los fosfolípidos de huevo para perfusión.
Los demás componentes son:
Ácido L-Málicoa |
Ácido clorhídricoa |
Fosfolípidos de huevo para perfusión |
Glicerol |
Oleato de sodio |
Hidróxido de sodioa |
Agua para preparaciones inyectables |
a para el ajuste del pH
Baxter y Numeta son marcas registradas de Baxter International Inc.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NUMETA G16%E, EMULSION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 4,76 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 g / 3,5 g / 200 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 3,5 g / 200 g / 5,22 g / 1,88 g / 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 662 mg / 1,02 g / 4,76 g / 5,15 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 4,25 g / 300 g / 5,22 g / 1,54 g / 4,76 g / 1,53 g / 8,76 g / 4,08 g / 5,1 g / 6,2 g / 3,57 g / 3,4 g / 662 mg / 1,02 g / 5,78 g / 5,94 g / 6,16 g / 4,93 g / 17,6 g / 0,34 g / 9,78 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere receta
Médicos online para NUMETA G16%E, EMULSION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NUMETA G16%E, EMULSION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















