
KENTERA 3,9 mg/24 HORAS, PARCHE TRANSDERMICO


Cómo usar KENTERA 3,9 mg/24 HORAS, PARCHE TRANSDERMICO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Kentera 3,9 mg / 24 horas, parche transdérmico
oxibutinina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Kentera y para qué se utiliza
- Qué necesita saber antes de empezar a usar Kentera
- Cómo usar Kentera
- Posibles efectos adversos
- Conservación de Kentera
- Contenido del envase e información adicional
1. Qué es Kentera y para qué se utiliza
Kentera se usa en adultos para controlar los síntomas de incontinencia de urgencia y/o del aumento de la frecuencia y urgencia miccionales.
Kentera funciona permitiendo que la vejiga se expanda y contenga más orina.
2. Qué necesita saber antes de empezar a usar Kentera
No useKentera
- si es alérgico a la oxibutinina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene una enfermedad rara llamada miastenia grave que hace que los músculos del cuerpo se vuelvan débiles y que se canse con facilidad.
- si al orinar su vejiga no se vacía completamente, la utilización de oxibutinina puede agravar el problema. Debe comentarlo con su médico antes de utilizar Kentera.
- si tiene problemas digestivos a causa de una reducción del vaciado del estómago después de las comidas, debe consultar a su médico antes de utilizar Kentera.
- si sufre de glaucoma o tiene antecedentes familiares de glaucoma, informe a su médico.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Kentera, si usted padece cualquiera de lo siguiente.
- Problemas hepáticos
- Problemas renales
- Dificultad para orinar
- Obstrucción intestinal
- Presencia de sangre en las heces
- Debilidad muscular generalizada
- Dolor al tragar
Como el tratamiento con oxibutinina puede reducir la sudoración, el riesgo de fiebre y golpe de calor es mayor cuando la temperatura ambiental es más alta.
Niños y adolescentes
El uso de Kentera no está recomendado en niños o adolescentes.
Otros medicamentos y Kentera
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamentos.
Utilizar el parche Kentera al mismo tiempo que se toman otros medicamentos que tengan efectos adversos similares, como la sequedad de boca, el estreñimiento y la somnolencia, puede aumentar la frecuencia o la gravedad de estos efectos adversos.
La oxibutinina puede enlentecer el funcionamiento del tubo digestivo e influir así en la adsorción de otros medicamentos orales; por otra parte, el uso de este fármaco junto con otros medicamentos puede aumentar el efecto de la oxibutinina, especialmente:
- Ketoconazol, itraconazol o fluconazol (usados para el tratamiento de las infecciones fúngicas).
- Eritromicina, un antibiótico macrólido (usado para tratar las infecciones bacterianas).
- Biperideno, levodopa o amantadina (usados para tratar la enfermedad de Parkinson).
- Antihistamínicos (usados en el tratamiento de alergias como la rinitis alérgica primaveral).
- Fenotiazinas o clozapina (usadas para tratar las enfermedades mentales).
- Antidepresivos tricíclicos (usados para tratar la depresión).
- Dipiridamol (usado para tratar los problemas de coagulación sanguínea).
- Atropina y otros anticolinérgicos (usados para tratar los trastornos estomacales como el síndrome del colon irritable).
Uso deKenteracon alcohol
La oxibutinina puede causar somnolencia y visión borrosa. El consumo de alcohol puede aumentar la somnolencia.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Kentera no debería utilizarse durante el embarazo excepto si fuese claramente necesario.
Cuando se utiliza oxibutinina durante la lactancia, se excreta una pequeña cantidad en la leche de la madre. Por lo tanto, no se recomienda el uso de oxibutinina durante la lactancia.
Conducción y uso de máquinas
Dado que Kentera puede provocar sopor, somnolencia o visión borrosa, se debe recomendar a los pacientes que tengan precaución a la hora de conducir y utilizar máquinas.
3. Cómo usar Kentera
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Aplique un parche de Kentera nuevo dos veces por semana (cada 3 o 4 días) en la forma indicada. Cambie el parche siempre los mismos dos días, por ejemplo los domingos y los miércoles, o los lunes y los jueves.
En la parte interna del envase de Kentera encontrará un calendario que le ayudará a recordar cuándo le toca su dosis. Marque los días en que ha decidido aplicar el medicamento y no olvide cambiarse el parche siempre en los mismos dos días de la semana que haya elegido. Asegúrese de que solo tiene un parche en el cuerpo cada vez, y manténgalo aplicado permanentemente hasta el momento en que tenga que cambiarlo por uno nuevo.
Dónde se aplica
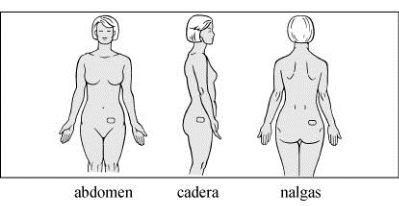
Aplicar el parche sobre una zona limpia, seca y lisa de la piel del abdomen, la cadera o las nalgas. No se recomienda aplicar el parche en la cintura, por el riesgo de roce contra la ropa ceñida. No exponer al sol. Colocar el parche bajo la ropa. Alternar los lugares de aplicación en cada nueva aplicación. No volver a aplicar otro parche en el mismo sitio durante al menos una semana.
Cómo se aplica
Cada parche se envuelve separadamente en un sobre protector. Lea las siguientes instrucciones antes de aplicar Kentera por primera vez.
Para aplicarKentera
Paso 1: Elegir un lugar apropiado para aplicar el parche.
- Piel recién lavada, pero seca y fresca (espere unos minutos después de un baño o ducha calientes).
- Donde no haya aplicado talco, lociones ni aceites corporales.
- Donde no tenga cortes, erupciones ni otras formas de irritación de la piel.
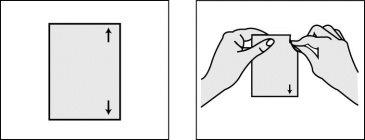
Paso 2: Abrir el sobre que contiene el parche.
- Rompa la bolsa por las flechas situadas en el lado derecho del sobre, como se indica en el dibujo.
- No utilice tijeras para cortar la bolsa: podría dañar el parche
- Retire el parche de la bolsa.
- No corte ni divida el parche. No utilice parches dañados.
- Aplíquelo inmediatamente sobre la piel; no conserve el parche fuera del sobre hermético.
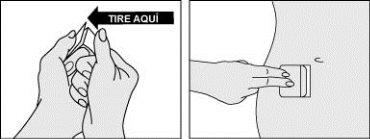
Paso 3: Aplicar la mitad del parche sobre la piel.
- Doble con cuidado el parche y desprenda una mitad del protector que recubre la superficie adhesiva del parche.
- Sin tocar la superficie adhesiva, aplique la parte adhesiva en la piel del lugar seleccionado en el abdomen, la cadera o las nalgas y haga presión.
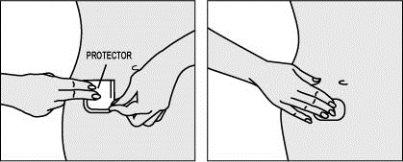
Paso 4: Aplicar la otra mitad del parche sobre la piel.
- Doble el parche sobre sí mismo, apretando sobre el recubrimiento.
- Tire ligeramente del recubrimiento para levantar el borde.
- Sujete el borde por una de sus esquinas y retire la segunda mitad del protector. Intente no tocar la parte adhesiva del parche.
- Haga presión con los dedos sobre todo el parche durante 10 segundos como mínimo para fijarlo bien. Asegúrese de que todo el parche queda adherido a la piel, incluso en los bordes.
- Deseche los protectores de recubrimiento.
Baño, ducha, natación y ejercicio
Debe llevar el parche continuamente hasta que se aplique uno nuevo. El baño, la ducha, la natación y el ejercicio no afectarán al parche siempre y cuando no lo frote cuando se lave. Evite permanecer en la bañera durante un período largo de tiempo, puesto que podría despegarse el parche.
Si el parche se despega
Si el parche empieza a separarse de la piel, aplique una presión ligera con los dedos. El parche ha sido diseñado para volver a adherirse. En raras ocasiones el parche se puede despegar completamente. En ese caso, intente volver a poner ese parche en el mismo lugar. Si todo el parche se adhiere firmemente, déjeselo puesto. Si no, retírelo y ponga un parche nuevo en un lugar diferente. Independientemente del día en que ocurra, siga con la misma pauta de dos veces por semana que tiene marcada en la caja de los parches.
Si se olvida de cambiar el parche después de 3-4días
En cuanto se acuerde, retire el parche viejo y aplique uno nuevo en un lugar distinto de su abdomen, cadera o nalgas. Independientemente del día en que ocurra, siga con la misma pauta de dos veces por semana para su próximo parche, incluso aunque deba cambiarlo antes de que hayan pasado 3 o 4 días.
Cómo debe retirarlo
Para cambiarlo, retire lentamente el parche usado. Dóblelo en dos (con la superficie adhesiva hacia dentro) y deséchelo de forma que quede fuera del alcance de los niños y los animales domésticos. El lugar de aplicación puede quedar ligeramente enrojecido, pero el enrojecimiento debe desaparecer unas horas después de retirar el parche. Consulte a su médico si la irritación persiste.
Normalmente, los restos de adhesivo del parche retirado se pueden eliminar lavando delicadamente la piel con agua tibia y un jabón suave. También pueden limpiarse con un poco de aceite para bebé. Para eliminar las marcas de adhesivos anteriores que hayan quedado puede ser necesario utilizar una toallita especial para limpiar restos de esparadrapo (en farmacias). No utilice alcohol ni otros disolventes fuertes que pueden irritar la piel.
Después de usarlo, el parche todavía contiene cantidades importantes de principios activos que pueden resultar nocivos para el medio acuático. Por tanto, después de retirarlo, el parche usado debe doblarse por la mitad, con la cara adhesiva hacia dentro para que la membrana de liberación no quede expuesta, colocarse en su sobre original y luego desecharse de forma segura y fuera del alcance de los niños. Todos los parches, tanto usados como sin utilizar, deberán eliminarse de acuerdo con la normativa local o devolverse a la farmacia. Los parches usados no deben tirarse al inodoro, ni desecharse en sistemas de eliminación de residuos líquidos.
Si usa más Kentera del que debe
No debe aplicarse más de un parche en el cuerpo cada vez.
Si olvidó usar Kentera
Aplíquese un parche Kentera tan pronto como se dé cuenta de que no lo lleva, o si se ha saltado uno de los días marcados en el calendario.
Si interrumpe el tratamiento con Kentera
Su incontinencia de urgencia puede volver, y puede que sufra un aumento de la frecuencia urinaria si decide dejar de usar el parche. Siga usando Kentera mientras su médico no le indique lo contrario.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede tener efectos adversos, aunque no todas las personas los sufran.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- picor alrededor del lugar de aplicación
Efectos adversos frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- enrojecimiento o erupción en el lugar de aplicación
- sequedad de boca
- estreñimiento
- diarrea
- molestias estomacales
- dolor de estómago
- dolor de cabeza o somnolencia
- infecciones urinarias
- visión borrosa
- mareos
Efectos adversos poco frecuentes(pueden afectar hasta a 1 de cada 100 personas)
- infecciones fúngicas o del tracto respiratorio superior
- ansiedad
- confusión
- nerviosismo
- agitación
- dificultad para dormir
- palpitaciones
- sofocos
- dolor de espalda
- retención urinaria
- dificultad para orinar
- resfriado
- lesión accidental
Efectos adversos raros(pueden afectar hasta a 1 de cada 1.000 personas)
- reacción de pánico
- confusión mental
- alucinaciones
- desorientación
- deterioro de la memoria
- pérdida de memoria
- cansancio anormal
- mala concentración
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Kentera
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el sobre y la caja. La fecha de caducidad es el último día del mes que se indica.
No refrigerar o congelar.
Los parches usados se deben doblar por la mitad, con la cara adhesiva hacia dentro para que la membrana de liberación no quede expuesta, colocarlos en su sobre original y luego desecharlos de forma segura fuera del alcance de los niños. Todos los parches, tanto usados como sin utilizar, deberán eliminarse de acuerdo con la normativa local o devolverse a la farmacia. Los parches usados no deben tirarse al inodoro, ni desecharse en sistemas de eliminación de residuos líquidos.
6. Contenido del envase e información adicional
Composición de Kentera
- El principio activo es la oxibutinina.
Cada parche transdérmico libera 3,9 mg de oxibutinina cada 24 horas. Cada parche de 39 cm2 contiene 36 mg de oxibutinina.
- Los demás componentes son: triacetina y solución adhesiva acrílica. La oxibutinina, la triacetina y el adhesivo acrílico tienen una película de refuerzo de PET/EVA transparente y están forrados con un recubrimiento de liberación de poliéster siliconado.
Aspecto del producto y contenido del envase
Kentera es un parche transdérmico que se presenta en cajas de 2, 8 o 24 parches.
Cada parche está recubierto de una película de refuerzo protectora en el lado del parche que está cubierto con los principios activos. La película de refuerzo se debe retirar antes de la aplicación del parche.
Titular de la autorización de comercialización
Teva B.V.
Swensweg 5
2031 GA Haarlem
Países Bajos
Responsable de la fabricación
Merckle GmbH
Ludwig-Merckle-Straße 3
89143 Blaubeuren
Alemania
Teva Pharmaceuticals Europe B.V.
Swensweg 5
2031 GA Haarlem
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien Teva Pharma Belgium N.V./S.A./AG Tél/Tel: +32 38207373 | Lietuva UAB Teva Baltics Tel: +370 52660203 |
???????? ???? ????? ??? ???: +359 24899585 | Luxembourg/Luxemburg Teva Pharma Belgium N.V./S.A./AG Belgique/Belgien Tél/Tel: +32 38207373 |
Ceská republika Teva Pharmaceuticals CR, s.r.o. Tel: +420 251007111 | Magyarország Teva Gyógyszergyár Zrt. Tel: +36 12886400 |
Danmark Teva Denmark A/S Tlf: +45 44985511 | Malta Teva Pharmaceuticals Ireland L-Irlanda Tel: +44 2075407117 |
Deutschland ratiopharm GmbH Tel: +49 73140202 | Nederland Teva Nederland B.V. Tel: +31 8000228400 |
Eesti UAB Teva Baltics Eesti filiaal Tel: +372 6610801 | Norge Teva Norway AS Tlf: +47 66775590 |
Ελλ?δα Specifar A.B.E.E. Τηλ: +30 2118805000 | Österreich ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
España Laboratorios Gebro Pharma, S.A. Tel: +34 932058686 | Polska Teva Pharmaceuticals Polska Sp. z o.o. Tel: +48 223459300 |
France Teva Santé Tél: +33 155917800 | Portugal Teva Pharma - Produtos Farmacêuticos, Lda. Tel: +351 214767550 |
Hrvatska Pliva Hrvatska d.o.o. Tel: +385 13720000 | România Teva Pharmaceuticals S.R.L. Tel: +40 212306524 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 2075407117 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 15890390 |
Ísland Teva Pharma Iceland ehf. Sími: +354 5503300 | Slovenskárepublika TEVA Pharmaceuticals Slovakia s.r.o. Tel: +421 257267911 |
Italia Teva Italia S.r.l. Tel: +39 028917981 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 201805900 |
Κ?προς Specifar A.B.E.E. Ελλ?δα Τηλ: +30 2118805000 | Sverige Teva Sweden AB Tel: +46 42121100 |
Latvija UAB Teva Baltics filiale Latvija Tel: +371 67323666 | United Kingdom (Northern Ireland) Accord Healthcare Ireland Ltd. Ireland Tel: +353 214619040 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
- País de registro
- Precio medio en farmacia40.59 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KENTERA 3,9 mg/24 HORAS, PARCHE TRANSDERMICOForma farmacéutica: COMPRIMIDO, 5 mgPrincipio activo: oxybutyninFabricante: Cheplapharm Arzneimittel GmbhRequiere recetaForma farmacéutica: PREPARADO IRRIGACION VESICAL, 1 mg/mlPrincipio activo: oxybutyninFabricante: Farco-Pharma GmbhRequiere recetaForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 50 mgPrincipio activo: mirabegronFabricante: Laboratorios Alter S.A.Requiere receta
Médicos online para KENTERA 3,9 mg/24 HORAS, PARCHE TRANSDERMICO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KENTERA 3,9 mg/24 HORAS, PARCHE TRANSDERMICO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






