
ISOPLASMAL G SOLUTION FOR INFUSION


How to use ISOPLASMAL G SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Isoplasmal G Solution for Infusion
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any of the side effects, or if you notice any side effects not mentioned in this leaflet, please inform your doctor or pharmacist.
Contents of the Package Leaflet:
- What Isoplasmal G Solution for Infusion (hereafter referred to as Isoplasmal) is and what it is used for
- Before using Isoplasmal
- How to use Isoplasmal
- Possible side effects
- Storage of Isoplasmal
- Additional information
1. What Isoplasmal is and what it is used for
Isoplasmal is a nutritional supplement administered directly into a vein, containing essential substances for the body's functions.
It is used to cover protein, glucose, electrolyte, and fluid needs during limited fasting periods.
2. Before using Isoplasmal
Do not useIsoplasmal
If you are allergic (hypersensitive) to any of the components of Isoplasmal
If you have:
- any metabolic disorder (degradation) of amino acids
- advanced liver disorders
- kidney function disorder (renal insufficiency) with abnormal levels of residual nitrogen
- excess potassium in the blood (hyperkalemia)
- excess water in the body (hyperhydration)
- heart function disorder (manifest heart failure)
- acidosis
- diabetes mellitus
- excess glucose in the blood (hyperglycemia)
Isoplasmal should not be used in premature and newborn infants.
Be especially careful withIsoplasmal
Regularly check that fluid and electrolyte levels, acid balance, are correct.
Ensure adequate vitamin intake (especially vitamin B1)
The sole use of Isoplasmal can be extended up to 7 days. In case of longer nutritional disorders, the usage time can be increased.
Using other medicines
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those bought without a prescription.
Amino acid solutions like Isoplasmal should not be used as carrier solutions for other medicines. If mixed with other medicines, reactions may occur that could alter the solution.
Pregnancy and breastfeeding
During pregnancy, your doctor will decide, after assessing the potential benefits and risks of administering Isoplasmal, whether you can take this medicine.
Consult your doctor or pharmacist before using any medicine.
3. How to use Isoplasmal
This medicine will always be administered by healthcare personnel.
Your doctor will determine the amount you need daily. The maximum dose is 40 ml per kg of body weight and per day.
This medicine must be administered into a vein, drop by drop.
If you use moreIsoplasmalthan you should
This is unlikely to happen since your doctor will determine your daily doses. However, if you receive more Isoplasmal than you should or the solution is injected too quickly, you may lose some of the amino acids in your urine and may feel nausea, chills, or vomiting. These symptoms will disappear as soon as the administration is stopped or the speed is reduced.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Isoplasmal can cause side effects, although not everybody gets them.
In exceptional cases, and when Isoplasmal is administered too quickly, there may be loss of amino acids in the urine.
The following side effects are not due to the medicine but may occur in general when any substance used for injectable nutrition is administered, especially at the start.
The following side effects have been reported when using Isoplasmal:
Uncommon (affect 1 to 10 in every 1,000 patients)
Gastrointestinal disorders:
nausea and vomiting.
General disorders and administration site conditions:
headache, chills, and fever.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Isoplasmal
Keep out of the sight and reach of children.
Do not store above 25°C.
Keep the bags in the outer packaging to protect them from light.
Do not use Isoplasmal after the expiry date (after EXP) stated on the packaging. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Additional information
Composition ofIsoplasmal
The active ingredients are amino acids, glucose, and electrolytes.
The composition after mixing the two chambers is as follows:
From the upper chamber:
Bag of 1000 ml | Bag of 2000 ml | |
Per 860 ml | Per 1720 ml | |
Isoleucine | 1.440 g | 2.880 g |
Leucine | 2.520 g | 5.040 g |
Lysine hydrochloride | 2.770 g | 5.540 g |
Methionine | 0.600 g | 1.200 g |
Phenylalanine | 1.260 g | 2.520 g |
Threonine | 1.440 g | 2.880 g |
Tryptophan | 0.600 g | 1.200 g |
Valine | 1.920 g | 3.840 g |
Arginine | 2.580 g | 5.160 g |
Histidine | 1.620 g | 3.240 g |
Alanine | 3.720 g | 7.440 g |
Glycine | 2.100 g | 4.200 g |
Asparagine monohydrate | 0.310 g | 0.620 g |
Aspartic acid | 0.270 g | 0.540 g |
Glutamic acid | 2.700 g | 5.400 g |
Ornithine hydrochloride | 0.690 g | 1.380 g |
Proline | 2.100 g | 4.200 g |
Serine | 0.960 g | 1.920 g |
Tyrosine | 0.430 g | 0.860 g |
Acetylcysteine | 0.820 g | 1.640 g |
Potassium acetate | 1.970 g | 3.940 g |
Sodium acetate trihydrate | 1.290 g | 2.580 g |
Sodium hydroxide | 0.240 g | 0.480 g |
From the lower chamber:
Bag of 1000 ml | Bag of 2000 ml | |
Per 140 ml | Per 280 ml | |
Glucose monohydrate | 55.00 g | 110.00 g |
Sodium chloride | 0.470 g | 0.940 g |
Magnesium chloride hexahydrate | 0.310 g | 0.620 g |
Sodium phosphate monohydrate | 0.780 g | 1.560 g |
Zinc acetate dihydrate | 0.0176 g | 0.0352 g |
Electrolytes | mmol/l |
Sodium | 28.5 |
Potassium | 20 |
Magnesium | 1.5 |
Zinc | 0.08 |
Chloride | 28.8 |
Acetate | 29.6 |
Phosphate | 5 |
Amino Acids Total | 30.9 g/l |
Total Nitrogen | 4.7 g/l |
Protein Equivalent | 29.21 g/l |
Theoretical Osmolality | 648.5 mOsm/l |
pH | 5.0 –6.5 |
AAE/AAT | 0.36 |
Energy Value | ? 319 kcal/l |
The other components are: citric acid monohydrate and water for injectable preparations.
Appearance and packaging of the product
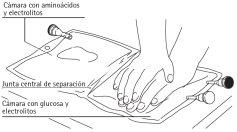
Isoplasmal is a clear, colorless or slightly amber-colored solution for infusion, presented in flexible plastic bags of 1000 ml and 2000 ml. The packaging is divided into two compartments, separated by an internal seal (stopper), of 860 ml and 140 ml or 1720 ml and 280 ml.
The design of the double-chamber bag allows for the mixing of amino acids and glucose. If necessary, lipids, vitamins, trace elements, and even more electrolytes can be added, provided that the solution does not precipitate.
Marketing authorization holder and manufacturer
- Braun Medical, S.A.
Ctra. de Terrassa, 121
08191-Rubí (Barcelona)
Spain
This leaflet was approved in February 2025.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products.
---------------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Single-use containers. Discard unused contents after completing the infusion.
Only use the solution if the container closure is not damaged and the solution is clear.
Use a sterile administration set for administration.
If it is necessary to add other nutrients such as carbohydrates, lipids, vitamins, and trace elements to this medicine in total parenteral nutrition, the addition should be performed under strict aseptic conditions. Mix well after adding any additive. Pay special attention to compatibility.
The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
Instructions for the correct use of Isoplasmal
Preparation:
After removing the protective cover, proceed as follows: Unfold the bag and place it on a hard surface. Open the central joint by pressing with both hands on one of the bag's chambers. The homogeneous mixture is ready for use. Remove the white cap from the infusion connector. Clean the connector and insert the infusion equipment. Infuse according to protocol.
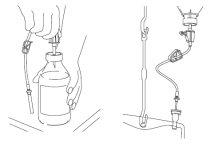
Adding lipids:
Close the regulation clamp and the air intake of the lipid transfer equipment. Disinfect the connectors and insert the spike of the transfer equipment into the lipid bottle and the needle into the addition connector located at the top of the bag. Ensure there is a distance of 40-60 cm (= 2-3 Medihook hangers) between the lipid bottle and the bag. Open the regulation clamp and the air intake of the transfer equipment. The lipid emulsion will flow into the bag by gravity. During lipid transfer, no supervision is necessary.
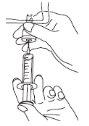
Additives:
If necessary, additives can be incorporated directly into the bag using a syringe in the connector located at the bottom of the bag, using an aseptic procedure. For information on compatibility, consult your pharmacist or the manufacturer/distributor.
Infusion:
Connect the infusion equipment (without air intake) to the infusion connector.
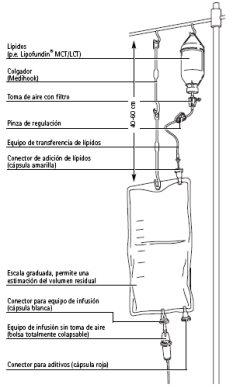
Mixing:
Hang the bag and mix the contents by inverting the bag 2-3 times.
The administration speed: maximum 40 drops per minute (120 ml/h).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ISOPLASMAL G SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for ISOPLASMAL G SOLUTION FOR INFUSION
Discuss questions about ISOPLASMAL G SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















