
REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL

Pergunte a um médico sobre a prescrição de REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL

Como usar REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL
Introdução
Prospecto: Informação para o utilizador
Reandron 1000mg / 4ml solução injetável
Testosterona, undecanoato
Leia todo o prospecto detenidamente antes de começar a usar este medicamentoporque contém informações importantes para si.
|
Conteúdo do prospecto
- O que é Reandron e para que é utilizado
- O que precisa saber antes de que lhe administrem Reandron
- Como usar Reandron
- Possíveis efeitos adversos
- Conservação de Reandron
- Conteúdo do envase e informações adicionais
1. O que é Reandron e para que é utilizado
Reandron contém testosterona, uma hormona masculina, como princípio ativo.
Reandron é administrado por injeção intramuscular; o medicamento administrado vai sendo libertado com o tempo.
Reandron é utilizado em homens adultos para o tratamento de substituição de testosterona e assim tratar diversos problemas de saúde derivados da falta de testosterona (hipogonadismo masculino). Isto deve ser confirmado mediante duas determinações separadas de testosterona no sangue e além disso apresentar sintomas clínicos como por exemplo:
- impotência
- infertilidade
- baixo apetite sexual
- cansaço
- estados de ânimo depressivos
- perda óssea causada por um nível hormonal baixo
2. O que precisa saber antes de que lhe administrem Reandron
Não use Reandron
- se é alérgico à testosterona undecanoato ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
- se padece cancro andrógeno dependente ou suspeita que pode padecer cancro de próstata ou de mama.
- se tem ou teve um tumor do fígado;
Reandron não está indicadopara uso em mulheres.
Advertências e precauções
Consulte o seu médicoantes de começar a usar Reandron se tiver ou alguma vez teve:
- epilepsia
- problemas de coração, fígado ou rim
- enxaqueca
- interrupções da respiração durante o sono (apneia) pois pode piorar com o tratamento
- cancro, pois deverá determinar os níveis de cálcio no sangue
- pressão arterial alta ou se está a ser tratado de hipertensão arterial, pois a testosterona pode causar um aumento da pressão arterial.
- problemas de coagulação
- distúrbios hemorrágicos (por exemplo hemofilia)
- trombofilia (anomalia da coagulação do sangue que aumenta o risco de trombose - coágulos de sangue nos vasos sanguíneos)
- fatores que aumentam o risco de coágulos sanguíneos em uma veia: coágulos sanguíneos previos em uma veia, fumar, obesidade, cancro, inatividade, se um familiar direto teve um coágulo de sangue em uma perna, pulmão ou outro órgão a uma idade precoce (por exemplo, aproximadamente abaixo dos 50 anos de idade), ou à medida que envelhece.
Como reconhecer um coágulo de sangue: inchaço doloroso de uma perna ou mudança repentina na cor da pele, por exemplo, tornar-se pálido, vermelho ou azul, dificuldade para respirar repentina, tosse repentina e inexplicável que pode provocar sangue, ou dor repentina no peito, tonturas ou vertigens severas, dor intensa no estômago, perda repentina da visão. Procure atendimento médico urgente se experimentar algum desses sintomas.
Se padece insuficiência grave do coração, fígado ou rim,o tratamento com Reandron pode causar-lhe complicações graves em forma de retenção de líquidos, que se pode acompanhar por vezes de insuficiência cardíaca (congestiva).
Antes de iniciar o tratamento e durante o mesmo, o seu médico comprovará os seguintes parâmetros no seu análise de sangue: nível de testosterona e hemograma completo.
Se o seu fígado não funciona bem
Não foram realizados estudos formais em pacientes com insuficiência hepática. Se alguma vez teve um tumor no fígado, não lhe será prescrito Reandron (ver “Não utilize Reandron”).
Crianças e adolescentes
Reandron nãoestá indicadopara uso em crianças e adolescentes. Não existe informação disponível sobre o uso de Reandron em varões menores de 18 anos.
Pacientes de idade avançada (65 anos ou mais)
Não é necessário ajustar a dose se tiver mais de 65 anos. (Ver “Exame médico/Seguimento”).
Musculação e testes de doping
Reandron nãoestá indicado para aumentar a musculatura em indivíduos saudáveis ou para incrementar a resistência física.
Reandron pode dar resultados positivos no teste de doping.
Abuso de substâncias e dependência
Utilize sempre este medicamento exatamente como o seu médico ou farmacêutico lhe indicou.
O abuso de testosterona, especialmente se você toma demasiada quantidade, seja deste medicamento sozinho ou em conjunto com outros esteroides anabólicos androgênicos, pode causar graves problemas de saúde ao seu coração e vasos sanguíneos (que lhe podem provocar a morte), à sua saúde mental e/ou ao fígado.
As pessoas que abusaram da testosterona podem tornar-se dependentes e podem experimentar sintomas de abstinência quando a dose muda de forma significativa ou quando se interrompe repentinamente o uso. Não deve abusar deste medicamento porque lhe pode ocasionar graves problemas de saúde, tanto se o utiliza sozinho como em combinação com outros esteroides anabólicos androgênicos. (ver “Possíveis efeitos adversos”)
Exame médico / Seguimento
As hormonas masculinas podem aumentar o crescimento do cancro da próstata ou aumentar o tamanho da glândula prostática (hipertrofia prostática benigna). Antes de iniciar o tratamento com Reandron, o seu médico deve realizar um exame médico, com o fim de excluir o risco de um cancro da próstata já existente.
O seu médico deve realizar um seguimento periódico cuidadoso da próstata e das mamas, especialmente em pacientes de idade avançada. O seu médico também lhe realizará periodicamente alguns análises de sangue.
Foram comunicadas a ocorrência de tumores do fígado benignos (não cancerosos) e malignos (cancerosos) em pacientes que foram tratados com produtos hormonais como compostos androgênicos.
Uso de Reandron com outros medicamentos
Informa o seu médico ou farmacêuticose está a utilizar ou utilizou recentemente ou pode ter que utilizar qualquer outro medicamento, mesmo os adquiridos sem receita médica. O seu médico pode ter que ajustar a dose se está a tomar outros medicamentos, como por exemplo:
- A hormona adrenocorticotrópica (ACTH) ou corticosteroides (usados para tratar diversas doenças, tais como reumatismo, artrite, reações alérgicas e asma). Reandron pode aumentar o risco de retenção de líquidos, especialmente se tiver problemas de coração ou de fígado.
- Medicamentos que fazem com que o sangue seja mais fluido (anticoagulantes orais, derivados cumarínicos) pois podem aumentar o risco de sangramento. O seu médico comprovará a sua dose.
- Medicamentos para o tratamento da diabetes. Pode ser necessário ajustar a dose do seu medicamento antidiabético. Como outros andrógenos, a testosterona pode aumentar o efeito da insulina.
Informa o seu médico se sofre alguma alteração da coagulação,pois é importante que o seu médico conheça esta informação para decidir se pode ser tratado com Reandron.
Reandron pode afetar também os resultados de testes de laboratório (por exemplo, da glândula tireoide). Informa ao médico ou ao pessoal do laboratório de que está em tratamento com Reandron.
Gravidez e amamentação
Reandron não está indicado para uso em mulheres e não deve ser usado em mulheres grávidas ou em período de amamentação.
Fertilidade
O tratamento com altas doses de medicamentos com testosterona podem interromper ou reduzir a produção de espermatozoides de forma reversível (ver também “Possíveis efeitos adversos”).
Condução e uso de máquinas
Não se observou nenhum efeito sobre a capacidade para conduzir e utilizar máquinas.
Reandron contém benzoato de benzilo
Este medicamento contém 2000 mg de benzoato de benzilo em cada 4 ml ampola/frasco equivalente a 500 mg/ml.
3. Como usar Reandron
O seu médico lhe injetará Reandron (1 ampola/frasco) por via intramuscular, muito lentamente. O tratamento será repetido às 10-14 semanas, tempo suficiente para manter os níveis de testosterona sem que esta se acumule no sangue.
Reandron deve ser administrado apenas por via intramuscular. Deve ter especial cuidado para não injetar o produto num vaso sanguíneo (ver “Administração”).
Início do tratamento
Antes de começar o tratamento e durante a primeira parte do mesmo, o seu médico determinará os seus níveis de testosterona no sangue. O seu médico pode administrar-lhe uma segunda injeção, como muito cedo, passadas 6 semanas desde a primeira injeção, com o fim de alcançar rapidamente o nível necessário de testosterona. Isto dependerá dos seus sintomas e dos seus níveis de testosterona.
Manutenção dos níveis de Reandron durante o tratamento
O intervalo entre injeções deve manter-se dentro do limite recomendado de 10 a 14 semanas. O seu médico medirá os seus níveis de testosterona de forma regular ao finalizar um intervalo de injeções para assegurar que os níveis são correctos. Se os níveis forem muito baixos, o seu médico pode aumentar a frequência das injeções. Se os níveis forem muito altos, o seu médico pode diminuir a frequência das injeções. Lembre-se dos dias de tratamento, pois de outra forma os seus níveis de testosterona não estarão correctamente controlados.
Se pensa que o efeito de Reandron é demasiado intenso ou muito débil, informe o seu médico.
Se usa mais Reandron do que deve
Os sintomas de ter recebido demasiado Reandron incluem:
- Irritabilidade
- Nervosismo
- Aumento de peso
- Ereções frequentes ou de longa duração
Informe o seu médico se tiver algum dos sintomas anteriores. O seu médico reduzirá a frequência das injeções ou suspenderá o tratamento.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
As reações adversas mais frequentessão acne e dor no local de aplicação da injeção.
Efeitos adversos frequentes(podem afectar até 1 de cada 10 pessoas)
- níveis anormalmente elevados de glóbulos vermelhos
- aumento de peso
- sofocos
- acne
- agrandamento da próstata e problemas associados
- reações diversas no local de administração da injeção, como por exemplo, dor, hematoma ou irritação
Efeitos adversos pouco frequentes(podem afectar até 1 de cada 100 pessoas)
- reação alérgica
- aumento do apetite, alterações em alguns resultados do análise de sangue, como por exemplo, aumento do açúcar ou das gorduras
- depressão, alterações emocionais, insónia, inquietude, agressividade ou irritabilidade
- dor de cabeça, enxaqueca ou tremores
- alterações cardiovasculares, pressão arterial elevada ou tonturas
- bronquite, sinusite, tosse, respiração entrecortada, roncos ou alterações da voz
- diarreia ou náusea
- alterações nos resultados das provas hepáticas
- perda de cabelo ou diversas reações cutâneas (por exemplo picar, vermelhidão ou pele seca)
- dor nas articulações ou nas extremidades, problemas musculares (por exemplo espasmos, dor ou rigidez) ou creatina fosfoquinase aumentada no sangue
- alterações do conducto urinário (por exemplo, diminuição do fluxo de urina, retenção de urina, necessidade urgente de urinar durante a noite)
- alterações da próstata (por exemplo, displasia prostática, endurecimento ou inflamação da próstata), alterações do apetite sexual, dor de testículos, dor, endurecimento ou agrandamento das mamas ou aumento do nível de hormonas masculinas e femininas
- cansaço, fraqueza generalizada, suor excessivo ou suor noturno
Efeitos adversos raros(podem afectar até 1 de cada 1.000 doentes)
- O líquido oleoso de Reandron pode alcançar os pulmões (microembolia pulmonar das soluções oleosas) e em raros casos pode provocar sinais e sintomas tais como tosse, respiração entrecortada, malestar geral, suor excessivo, dor no peito, tonturas ou vertigens severas, dor intensa no estômago, perda repentina da visão. Procure atendimento médico urgente se experimentar algum desses sintomas.
Foram notificadas suspeitas de reações anafilácticas após a injeção de Reandron.
Além dos referidos anteriormente, foram observados os seguintes efeitos adversos após o tratamento com produtos que contêm testosterona: nervosismo, hostilidade, breves interrupções da respiração durante o sono (apneia), reações na pele como por exemplo, caspa e seborreia, crescimento excessivo do pelo, ereções mais frequentes e em casos muito raros cor amarelo na pele e nos olhos (icterícia).
O tratamento com doses elevadas de testosterona geralmente interrompe ou reduz a produção de espermatozoides, embora volte à normalidade após interromper o tratamento. O tratamento de substituição de testosterona em caso de testículos de baixo funcionamento (hipogonadismo), pode provocar em raros casos ereções persistentes e dolorosas (priapismo). Os tratamentos de longa duração ou com doses altas de testosterona podem produzir ocasionalmente um aumento da retenção de líquidos e edema (inchaço devido à retenção).
Em geral, com os medicamentos à base de testosterona foi observado um risco frequente de aumento do recuento de glóbulos vermelhos, do hematocrito (porcentagem de glóbulos vermelhos no sangue) e da hemoglobina (o componente dos glóbulos vermelhos que transporta o oxigénio), identificados mediante análises de sangue periódicas.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informação sobre a segurança deste medicamento.
5. Conservação de Reandron
Mantenha este medicamento fora da vista e do alcance das crianças.
Este medicamento não requer condições especiais de conservação.
Não utilize este medicamento após a data de validade que aparece no envase após CAD. A data de validade é o último dia do mês que se indica.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os envases e os medicamentos que não precisa no ponto SIGRE da sua farmácia habitual. Pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Reandron
O princípio ativo é undecanoato de testosterona 250 mg/ml (correspondente a 157,9 mg de testosterona). 1 ampola/frasco contém 1.000 mg de undecanoato de testosterona (que correspondem a 631,5 mg de testosterona).
Os demais componentes são: benzoato de benzilo e óleo de rícino refinado.
Aspecto do produto e conteúdo do envase
Reandron é uma solução oleosa clara, entre incolor e marrom amarelada. Apresenta-se em ampolas de vidro de cor topázio/frascos de vidro de cor topázio, que contêm 4 ml de solução injetável.
Título da autorização de comercialização e responsável pela fabricação
Título da autorização de comercialização Grünenthal Pharma, S.A. Doutor Zamenhof, 36 28027 Madrid Espanha | Responsável pela fabricação Bayer AG 13342 Berlim Alemanha ou EVER Pharma Jena GmbH Brüsseler Strasse 18 07747 Jena Alemanha ou Grünenthal GmbH Zieglerstrasse 6 52078 Aachen Alemanha ou Grünenthal Pharmaceuticals GmbH & Co. KG Philipp-Ott-Straße 3 51373 Leverkusen Alemanha |
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
- Chipre, República Checa, Grécia, Dinamarca, Luxemburgo, Malta, Polônia e Portugal: Nebido
- Áustria: Nebido 1000 mg/4 ml Injektionslösung
- Bélgica: Nebido 1000 mg/4 ml, oplossing voor injectie
- Bulgária: ?????? 250 mg/ml ??????????? ???????
- Croácia: Nebido 1000 mg/4 ml otopina za injekcij
- Finlândia: Nebido 1000 mg/4 ml injektioneste, liuos
- França: Nebido 1000 mg/4 ml, solution injectable
- Alemanha: Nebido 1000 mg Injektionslösung
- Hungria: Nebido 250 mg/ml oldatos injekció
- Islândia: Nebido 1000 mg/4 ml stungulyf, lausn
- Itália: NEBIDO 1000 mg/4ml soluzione iniettabile
- Lituânia: Nebido 1000 mg/4 ml injekcinis tirpalas
- Holanda: Nebido 1000 mg/4 ml
- Noruega: Nebido 1000 mg/4 ml injeksjonsvæske, oppløsning
- Romênia: Nebido 1000 mg/ 4 ml solutie injectabila
- Eslováquia: Nebido 1000 mg/4 ml injekcný roztok
- Eslovênia: Nebido 1000 mg/4 ml raztopina za injiciranje
- Espanha: Reandron 1000 mg/4 ml solução injetável
- Suécia: Nebido, 1000 mg/4 ml injektionsvätska, lösning
- Reino Unido e Irlanda: Nebido 1000mg/4ml, solution for injection
Data da última revisão deste prospecto:Outubro 2022
A informação detalhada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/
--------------------------------------------------------------------------------------------------------------------
Esta informação está destinada unicamente a profissionais do setor sanitário:
A temperaturas de armazenamento em frio, as propriedades desta solução de base oleosa poderiam mudar temporariamente (por exemplo, maior viscosidade, turbidez). Se estiver armazenado a temperatura fria, o produto deve ser levado à temperatura ambiente ou temperatura corporal antes de usá-lo.
A solução para injeção intramuscular deve ser inspecionada de forma visual antes de seu uso, devendo ser usadas apenas aquelas soluções claras e livres de partículas.
O conteúdo de uma ampola/frasco deve ser injetado por via intramuscular imediatamente após abrir a ampola/frasco.
O medicamento é para um único uso, devendo ser descartado o resto da solução não utilizada.
Administração
Deve-se prestar muito cuidado para não injetar o medicamento em um vaso sanguíneo.
Como com todas as soluções oleosas, Reandron deve ser injetado unicamente por via intramuscular e muito lentamente. A microembolia pulmonar relacionada com as soluções oleosas pode provocar em raras ocasiões sinais e sintomas como tosse, dispnéia, malestar, hiperhidrose, dor no peito, tontura, parestesia ou síncope. Estas reações podem ocorrer durante ou imediatamente após a injeção e são reversíveis. O tratamento é normalmente de apoio, por exemplo, administração de oxigênio suplementar.
Foram comunicadas suspeitas de reação anafiláctica após a administração de Reandron.
Advertências
Deverá ser realizado um controle regular cuidadoso da glândula prostática e das mamas segundo os métodos estabelecidos (exame retal digital e determinação do PSA no soro) nos pacientes que recebem tratamento com testosterona, pelo menos uma vez ao ano e duas vezes ao ano nos pacientes de idade avançada e de risco (aqueles com fatores clínicos ou familiares).
Além dos testes de laboratório de determinação da concentração de testosterona no sangue nos pacientes em tratamento de longa duração, deverão ser realizados periodicamente os seguintes testes de laboratório: hemoglobina, hematocrito, testes da função hepática e perfil lipídico.
Em pacientes com insuficiência cardíaca, hepática ou renal grave ou com cardiopatia isquémica, o tratamento com testosterona pode causar complicações graves, caracterizadas por edema com ou sem insuficiência cardíaca congestiva. Neste caso, deve-se interromper o tratamento imediatamente.
Normas para abrir a ampola de Reandron com sistema de abertura de “Um ponto de corte” (UPC)
A ampola tem uma marca abaixo do ponto colorido. Antes de abrir, certifique-se de que não reste solução na parte superior da ampola. Use ambas as mãos para abri-la; segure com uma mão a parte inferior da ampola e use a outra mão para pressionar para fora e quebrar a parte superior da ampola em direção oposta ao ponto colorido.

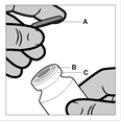
Normas para abrir o frasco de Reandron
O frasco é para um único uso. O conteúdo do frasco deve ser injetado por via intramuscular imediatamente após carregar a seringa. Após retirar a cobertura de plástico (A) não retire o anel metálico (B) nem a cobertura do rebordo (C).

Quanto custa o REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL em Espanha em 2025?
O preço médio do REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL em dezembro de 2025 é de cerca de 89.61 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia89.61 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVELForma farmacêutica: GEL, 2% testosteronaSubstância ativa: testosteroneFabricante: Advanz Pharma LimitedRequer receita médicaForma farmacêutica: GEL, 23 mg/gSubstância ativa: testosteroneFabricante: The Simple Pharma Company LimitedRequer receita médicaForma farmacêutica: INJETÁVEL, Propionato de Testosterona 25 mg/mlSubstância ativa: testosteroneFabricante: Desma Laboratorio Farmaceutico S.L.Requer receita médica
Alternativas a REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL em Poland
Alternativa a REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL em Ukraine
Médicos online para REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de REANDRON 1000 mg/4 ml SOLUÇÃO INJETÁVEL – sujeita a avaliação médica e regras locais.











