
MERIOFERT KIT 900 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL


Como usar MERIOFERT KIT 900 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Introdução
Prospecto: informação para o utilizador
Meriofert Kit 900 UIpó e diluente para solução injectável
Menotropina
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que o reler.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
- Neste prospecto, Meriofert Kit 900 UI de pó e diluente para solução injectável é denominado Meriofert Kit.
Conteúdo do prospecto
- O que é Meriofert Kit e para que é utilizado
- O que precisa saber antes de começar a usar Meriofert Kit
- Como usar Meriofert Kit
- Possíveis efeitos adversos
- Conservação de Meriofert Kit
- Conteúdo do envase e informações adicionais
1. O que é Meriofert Kit e para que é utilizado
- Meriofert Kit é usado para estimular a ovulação em mulheres que não ovulam e que não responderam a outro tratamento (citrato de clomifeno).
respondeu a outro tratamento (citrato de clomifeno).
- Meriofert Kit é usado para provocar o desenvolvimento de vários folículos (e, portanto, de vários óvulos) em mulheres submetidas a um tratamento de fertilidade.
Meriofert Kit é uma gonadotropina menopáusica humana muito purificada, que pertence a um grupo de medicamentos denominados gonadotropinas.
Cada frasco multidose contém pó liofilizado com 900 UI de actividade de hormona foliculoestimulante (FSH) humana e 900 UI de actividade de hormona luteinizante humana (LH).
A gonadotropina menopáusica humana (HMG) é extraída da urina de mulheres pós-menopáusicas. É adicionada gonadotropina coriónica humana (hCG), extraída da urina de mulheres grávidas, para contribuir para a actividade de LH total.
Este medicamento deve ser usado sob a supervisão de um médico.
2. O que precisa saber antes de começar a usar Meriofert Kit
Antes de começar o tratamento, será avaliada a sua fertilidade e a do seu parceiro.
Não use Meriofert Kit
- Se apresentar um aumento do tamanho dos seus ovários, ou quistes cuja causa não seja um distúrbio hormonal (doença ovárica poliquística)
- Se tiver sangramentos de origem desconhecida
- Se padece cancro dos ovários, útero ou mama
- Se apresentar uma inchação anormal (tumor) da hipófise ou do hipotálamo (cérebro)
- Se é alérgica à menotropina ou a qualquer um dos outros componentes deste
medicamento (listados na secção 6).
Não deve utilizar este medicamento se sofre de menopausa precoce, malformação dos órgãos genitais ou determinados tumores do útero que impediriam um embarazo normal.
Advertências e precauções
Embora não sejam descritas reacções alérgicas a Meriofert Kit, deverá informar o seu médico se padece alguma reacção alérgica a medicamentos semelhantes.
Este tratamento aumenta o risco de padecer uma doença denominada síndroma de hiperestimulação ovárica (SHO)(ver Possíveis efeitos adversos). Se se produzir uma hiperestimulação ovárica, será suspenso o tratamento e deverão ser evitados os embarazos. Os primeiros sinais de hiperestimulação ovárica são dor na região inferior do abdómen, náuseas (malestar), vómitos e ganho de peso. Se se produzirem ditos sintomas, deverá ser examinado por um médico o mais breve possível. Em casos graves, mas raros, podem aumentar os ovários e acumular-se líquido no abdómen ou no peito.
O medicamento empregado para provocar o desprendimento definitivo dos óvulos maduros (que contém hCG) pode aumentar a probabilidade de sofrer um SHO. Por tanto, não é recomendável utilizar hCG nos casos em que se está a produzir um SHO e não deverão manter relações sexuais durante um mínimo de 4 dias, embora se utilize um método anticonceptivo de barreira.
Cabe destacar que as mulheres com problemas de fertilidade têm uma taxa de abortos superior à da população normal.
A frequência de embarazos e partos múltiplos nas pacientes submetidas a um tratamento para estimular a ovulação é maior do que nas mulheres que concebem de forma natural. No entanto, este risco pode ser reduzido ao mínimo se for utilizada a dose recomendada.
O risco de embarazo ectópico (embarazo fora do útero) é ligeiramente maior nas mulheres com lesões nas trompas de Falópio.
Os embarazos múltiplos e as características dos progenitores submetidos a tratamentos de fertilidade (por exemplo, a idade da mãe ou as características do sêmen) podem estar associados a um aumento do risco de defeitos de nascimento.
O tratamento com Meriofert Kit, assim como o próprio embarazo, pode aumentar a probabilidade de sofrer uma trombose. A trombose é a formação de um coágulo de sangue num vaso sanguíneo, a maior parte das vezes nas veias das pernas ou nos pulmões.
Comente este facto com o seu médico antes de começar o tratamento, especialmente:
- Se já sabe que tem uma maior probabilidade de sofrer uma trombose.
- Se si ou algum familiar próximo sofreu alguma vez uma trombose.
- Se padece sobrepeso grave.
População pediátrica
O medicamento não é indicado para uso pediátrico.
Uso de Meriofert Kit com outros medicamentos
Informe o seu médico ou farmacêutico se está a tomar, tomou recentemente ou pode ter que tomar qualquer outro medicamento.
Embarazo, lactação e fertilidade
Meriofert Kit não deve ser utilizado se está grávida ou em período de amamentação.
Condução e uso de máquinas
A influência de Meriofert Kit sobre a capacidade para conduzir e utilizar máquinas é nula ou insignificante.
Meriofert Kit contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por dose; isto é, é essencialmente “isento de sódio”.
3. Como usar Meriofert Kit
Dose e duração do tratamento
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico.
Mulheres que não ovulam e têm períodos irregulares ou ausência de menstruação:
Por norma geral, a primeira injeção de um frasco de 75 UI de menotropina é administrada durante a primeira semana do ciclo após a menstruação espontânea ou induzida.
Posteriormente, é injectada todos os dias a dose deste medicamento prescrito pelo médico e continua-se com o tratamento até que sejam formados um ou mais folículos maduros no ovário. O médico ajustará a dose deste medicamento dependendo da resposta ovárica, que é determinada por meio de explorações clínicas.
Assim que um folículo atingir a fase de desenvolvimento necessária, será interrompido o tratamento com este medicamento e será provocada a ovulação com outra hormona (gonadotropina coriónica, hCG).
A ovulação produz-se, por norma, num prazo de entre 32 e 48 horas.
Nesta fase do tratamento existe a possibilidade de fertilização. Ser-lhe-á recomendado que mantenha relações sexuais todos os dias a partir do dia anterior à administração de hCG. Se, apesar da ovulação, não se consiga um embarazo, pode ser repetido o tratamento.
Mulheres submetidas a uma estimulação ovárica para o desenvolvimento folicular múltiplo antes de uma fertilizaçãoin vitroou outras técnicas de reprodução assistida
O objectivo deste método é obter um desenvolvimento folicular múltiplo simultâneo. O tratamento começará no segundo ou terceiro dia do ciclo com injeções de entre 150 e 300 UI de Meriofert Kit. O médico pode optar por administrar doses mais elevadas se for necessário. A dose deste medicamento que é injectada é maior do que a do método empregado para a fertilização natural. O médico se encarrega de ajustar a continuação do tratamento de forma individualizada.
Assim que se tenha desenvolvido um número suficiente de folículos, será interrompido o tratamento com este medicamento e será provocada a ovulação mediante a injeção de outra hormona (gonadotropina coriónica, hCG).
Como administrar Meriofert Kit:
Este medicamento é administrado sob a forma de injeção sob a pele (por via subcutânea).
Os frascos apenas devem ser reconstituídos uma vez e cada injeção única deve ser administrada tão pronto quanto a dose necessária seja extraída.
Depois de aconselhá-lo e instruí-lo devidamente, é possível que o médico lhe peça que se administre a injeção de Meriofert Kit si mesmo.
Antes da primeira injeção, o seu médico deve:
- Permitir-lhe que pratique a autoadministração de uma injeção subcutânea
- Indicar-lhe os locais onde pode administrar a injeção
- Ensinar-lhe a preparar a solução injectável
- Explicar-lhe como preparar a dose correcta para a injeção.
Antes de administrar a injeção de Meriofert Kit, leia atentamente as seguintes instruções.
Como este frasco contém medicação para vários dias de tratamento, deve ter a certeza de que apenas extrai a quantidade de medicação que o seu médico prescreveu. O seu médico prescreveu uma dose de Meriofert Kit em UI (unidades). Para obter a dose correcta, deve utilizar uma das 12 seringas de administração graduadas em unidades UI de FSH/LH que são fornecidas no envase.
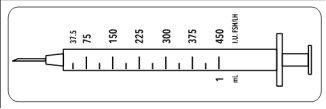
Estas seringas descartáveis destinam-se a um único uso e devem ser descartadas após a administração, de acordo com a regulamentação local, num contentor adequado.
Como preparar e injectar 1 frasco de Meriofert Kit:
A solução injectável que contém 900 UI de menotropina deve ser preparada exactamente antes de que esteja pronto para administrar a primeira dose. Para isso, adicione ao frasco que contém o pó o diluente para reconstituição da seringa pré-carregada (incluída no envase).
Prepare uma superfície limpa e lave as mãos com sabão e água morna. É importante que as suas mãos e os artigos que utilize estejam o mais limpos possível.
Coloque sobre a superfície os seguintes artigos:
- o frasco de pó de Meriofert Kit
- a seringa pré-carregada com diluente para a reconstituição
- a agulha para preparar a reconstituição
- uma seringa descartável de administração subcutânea com agulha pré-fixada graduada em unidades FSH/LH
- um cotonete com álcool
- algodão e solução desinfectante (não incluídos no envase)
LEMBRE-SE:
- Desinfectar o tampão de borracha do frasco que contém a solução reconstituída com algodão e desinfectante (isto é, solução alcoólica) e deixar secar antes da reconstituição e de cada administração.
- Não retire a lingueta de suporte (peça branca) da seringa pré-carregada, pois evita a extracção involuntária do pistão e melhora o manuseio da seringa durante a injeção.
Reconstituição da solução injectável
Preparação da seringa pré-carregada:
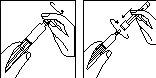 1.
1.
- Retire o capuchão da seringa pré-carregada com diluente; acople a agulha de reconstituição com o tampão protector ainda na seringa.
- Coloque com cuidado a seringa sobre uma superfície limpa.
Preparação do frasco:
 2.
2.
- Retire o tampão abatível de plástico colorido do frasco empurrando-o suavemente com o polegar para cima.
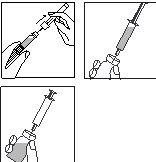 Limpe a zona do tampão de borracha com um cotonete e uma solução desinfectante e deixe secar.
Limpe a zona do tampão de borracha com um cotonete e uma solução desinfectante e deixe secar.
3.
- Pegue na seringa, retire o capuchão protector da agulha e pressione a agulha através do centro do tampão de borracha do frasco.
- Empurre com firmeza o êmbolo para esvaziar toda a solução sobre o pó.
- Ao adicionar o diluente, cria-se uma ligeira sobrepresão no frasco. Por isso, solte o êmbolo da seringa para que suba por si mesmo durante uns 10 segundos. Isso eliminará a sobrepresão no frasco.
NÃO AGITE a solução reconstituída, faça-a girar suavemente até obter uma solução transparente. Por norma, o medicamento dissolve-se imediatamente.
Verifique que a solução reconstituída é transparente.
Antes da injeção:
- Verifique que a solução reconstituída seja transparente, incolor e sem partículas. NÃO USE se a solução contém partículas, está turva ou não é incolor.
- Limpe a zona do tampão de borracha com um cotonete e uma solução desinfectante.
Preparação da injeção:
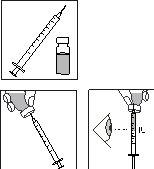 4.
4.
- Pegue numa das seringas descartáveis com agulha pré-fixada, retire o capuchão protector da agulha e insira a agulha verticalmente no centro da parte superior do frasco.
- Empurre o êmbolo até que esteja completamente pressionado.
- Inverta o frasco. Certifique-se de que a agulha está debaixo da superfície do medicamento e extraia a dose prescrita de medicamento na seringa de administração.
- Retire a agulha do frasco. Segure a seringa com a agulha apontando para cima e bata suavemente no lado da seringa para forçar qualquer bolha de ar para cima.
- Empurre o êmbolo lentamente até que apareça uma gota de líquido na ponta da agulha.
LEMBRE-SE: devido a que o frasco contém medicação para vários dias de tratamento, deve ter a certeza de que apenas retira a quantidade de medicação que o seu médico prescreveu.
Administração da injeção
Zona de injeção:
 O seu médico ou enfermeiro já lhe explicou em que parte do corpo deve injectar o medicamento. Os locais habituais são a coxa ou a parede inferior do abdómen abaixo do umbigo.
O seu médico ou enfermeiro já lhe explicou em que parte do corpo deve injectar o medicamento. Os locais habituais são a coxa ou a parede inferior do abdómen abaixo do umbigo.
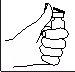
- Limpe a zona de injeção com um cotonete com álcool.
- Pegue e aperte com firmeza a pele. Com a outra mão, introduza a agulha com um movimento seco e rápido, formando um ângulo de 45° ou 90°.
Injeção da solução:
- Injete a seringa sob a pele tal como lhe indicaram. Não a injete directamente numa veia. Empurre o êmbolo lentamente e sem interrupções, para que a solução seja injectada correctamente e os tecidos cutâneos não sofram danos.
Leve o tempo que necessário para injectar o volume de solução que lhe foi prescrito.
Retire rapidamente a agulha e pressione a zona de injeção com um cotonete com desinfectante. Massageie suavemente a zona (enquanto mantém a pressão), isso ajuda a dispersar o medicamento e alivia as possíveis molestias.
Próximas injeções:
Repita desde o passo 4 em diante para as próximas injeções com a solução reconstituída de Meriofert Kit.
Se usar mais Meriofert Kit do que deve
Desconhecem-se os efeitos da sobredose deste medicamento, no entanto, caberia esperar que se produzisse um síndroma de hiperestimulação ovárica (ver Possíveis efeitos adversos). Se usar mais medicamento do que deve, consulte o seu médico ou enfermeiro.
Se esquecer de usar Meriofert Kit
Use-o no prazo em que lhe tocaria normalmente a próxima injeção. Não tome uma dose dupla para compensar as doses esquecidas.
Se interromper o tratamento com Meriofert Kit
Não o interrompa por iniciativa própria. Consulte sempre o seu médico antes de deixar de tomar este medicamento.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
O seguinte efeito adverso é importante e exigirá uma intervenção imediata se o sofrer. Deverá deixar de tomar este medicamento e acudir ao médico imediatamente se se produzir o seguinte:
Frequentes (pode afectar até 1 de cada 10 pessoas):
- Síndroma de hiperestimulação ovárica (os sintomas incluem a formação de quistes ováricos ou o aumento do tamanho de quistes existentes, dor na parte inferior do estômago, sensação de sede e náuseas com vómitos ocasionais, evacuação de pequenas quantidades de urina concentrada e ganho de peso) (ver secção 2 para obter informações adicionais).
Também foram comunicados os seguintes efeitos adversos:
Muito frequentes (podem afectar mais de 1 de cada 10 pessoas):
- Cefaleia
- Inchação do estômago ou meteorismo
Frequentes (podem afectar até 1 de cada 10 pessoas):
- Dor ou molestia abdominal
- Dor pélvica
- Dor de costas
- Sensação de peso
- Molestia na mama
- Tontura
- Sofocos
- Sede
- Sensação de doença
- Cansaço
- Malestar geral
- Reacções na zona de injeção, tais como dor e inflamação
Raros (podem afectar até 1 de cada 1.000 pessoas):
- Torsão ovárica (rotação do ovário que causa uma dor de grande intensidade na parte inferior do abdómen)
- Tromboembolia (formação de um coágulo num vaso sanguíneo que se desprende e é transportado pelo torrente sanguíneo para bloquear outro vaso).
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Meriofert Kit
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no embalagem exterior, no frasco e na seringa pré-carregada de solvente após CAD. Se a data de validade for indicada como mês/ano, a data de validade é o último dia do mês indicado.
Antes da reconstituição: Conservar na geladeira (entre 2°C e 8°C).
Após a reconstituição, a solução pode ser conservada durante um máximo de 28 dias a não mais de 25°C.
Não congelar antes ou após a reconstituição.
Não utilize este medicamento se observar que a solução não é transparente. Após a reconstituição, a solução deve ser transparente e incolor.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os recipientes e os medicamentos que não precisa no Ponto SIGRE  da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos recipientes e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos recipientes e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Meriofert Kit
O princípio ativo é menotropina.
Cada frasco multidose contém pó liofilizado com 900 UI de atividade de hormona foliculoestimulante (FSH) humana e 900 UI de atividade de hormona luteinizante (LH) humana.
A gonadotropina menopáusica humana (HMG) é extraída da urina de mulheres pós-menopáusicas. É adicionada gonadotropina coriônica humana (hCG), extraída da urina de mulheres grávidas, para contribuir para a atividade de LH total.
Os demais componentes são
Pó: lactose monoidratada, polissorbato 20, dihidrogenofosfato de sódio diidratado, ácido fosfórico e hidróxido de sódio.
Solvente: metacresol e água para preparações injetáveis.
Aspecto de Meriofert Kit e conteúdo do envase
Pó: torta ou pó branco liofilizado.
Solvente: solução transparente e incolor.
Meriofert Kit é apresentado como pó e solvente para solução injetável.
Um estojo contém:
- 1 frasco de pó de Meriofert Kit
- 1 seringa pré-carregada com solvente para a reconstituição
- 1 agulha para a reconstituição
- 12 cotonetes com álcool para as injeções múltiplas
- 12 seringas descartáveis com agulhas pré-fixadas para as injeções múltiplas
Título da autorização de comercialização e responsável pela fabricação
Título da autorização de comercialização
IBSA FARMACEUTICI ITALIA SRL
Via Martiri di Cefalonia 2
26900 Lodi, Itália
Responsável pela fabricação
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Itália
ou (para Reino Unido/Irlanda do Norte)
IBSA Pharma Limited
Unidades 4-6
Colonial Business Park
Colonial Way
Watford D24 4PR
Reino Unido
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Instituto Bioquímico Ibérico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (Espanha)
Este medicamento está autorizado nos estados membros do Espaço Econômico Europeu e no Reino Unido (Irlanda do Norte) com os seguintes nomes (as concentrações e formas farmacêuticas são idênticas em todos os países, apenas mudam os nomes comerciais):
Áustria: Meriofert PFS
Bélgica: Fertinorm Kit
Bulgária: Meriofert PFS
Chipre: Meriofert PFS
República Checa: Meriofert Set
Dinamarca: Meriofert Set
Estônia: Meriofert Set
Finlândia: Meriofert Set
França: Fertistartkit
Grécia: Meriofert
Hungria: Meriofert Kit
Itália: Meriofert
Letônia: Meriofert Set
Lituânia: Meriotert Set
Luxemburgo: Fertinorm Kit
Noruega: Meriofert Set
Polônia: Mensinorm Set
Romênia: Meriofert PFS
Eslováquia: Meriofert Kit
Espanha: Meriofert Kit
Suécia: Meriofert Set
Países Baixos: Meriofert spuit
Reino Unido: Meriofert PFS
Data da última revisão deste prospecto: Maio 2024
A informação detalhada e atualizada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a MERIOFERT KIT 900 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVELForma farmacêutica: INJETÁVEL, -Substância ativa: human menopausal gonadotrophinFabricante: Angelini Pharma Espana S.L.Requer receita médicaForma farmacêutica: INJETÁVEL, 1200 UISubstância ativa: human menopausal gonadotrophinFabricante: Ferring S.A.Requer receita médicaForma farmacêutica: INJETÁVEL, 1200 UISubstância ativa: human menopausal gonadotrophinFabricante: Ferring S.A.U.Requer receita médica
Alternativas a MERIOFERT KIT 900 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a MERIOFERT KIT 900 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL em Poland
Alternativa a MERIOFERT KIT 900 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL em Ukraine
Médicos online para MERIOFERT KIT 900 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de MERIOFERT KIT 900 UI PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL – sujeita a avaliação médica e regras locais.





