

DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL

Pergunte a um médico sobre a prescrição de DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL

Como usar DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL
Introdução
Prospecto: informação para o paciente
Dymista
137 microgramas/50 microgramas/ aplicação,
suspensão para pulverização nasal
Hidrocloruro de azelastina / propionato de fluticasona
Leia todo o prospecto detenidamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo neste prospecto:
- O que é Dymista e para que é utilizado.
- O que precisa saber antes de começar a usar Dymista.
- Como usar Dymista.
- Possíveis efeitos adversos.
- Conservação de Dymista.
- Conteúdo do envase e informações adicionais.
1. O que é Dymista e para que é utilizado
Dymista contém duas substâncias ativas: hidrocloruro de azelastina e propionato de fluticasona.
- O hidrocloruro de azelastina pertence a um grupo de medicamentos chamados antihistamínicos. Os antihistamínicos actuam prevenindo os efeitos de substâncias como a histamina, produzidas pelo organismo como parte de uma reação alérgica; desta forma, reduz os sintomas da rinite alérgica.
- O propionato de fluticasona pertence a um grupo de medicamentos chamados corticosteroides, que reduzem a inflamação.
Dymista é utilizado para aliviar os sintomas da rinite alérgica sazonal de moderada a grave e da rinite alérgica perene, se o tratamento com antihistamínico ou corticosteroide intranasal por separado for considerado insuficiente.
A rinite alérgica sazonal ou perene são reações alérgicas a substâncias como o pólen (febre do feno), ácaros domésticos, bolores, poeira ou animais de estimação.
Dymista alivia os sintomas das alergias, por exemplo: rinorreia, gotejamento pós-nasal, espirros, coceira ou entupimento nasal.
2. O que precisa saber antes de começar a usar Dymista
Não use Dymista:
? Se for alérgico ao hidrocloruro de azelastina ou ao propionato de fluticasona, ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6).
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a usar Dymista se:
? Teve uma operação recente no nariz.
? Padece uma infecção nasal. As infecções nasais devem ser tratadas com antibacterianos ou antifúngicos. Se lhe foi prescrito medicamento para uma infecção nasal, pode continuar a usar Dymista para tratar a sua alergia.
? Tem tuberculose ou uma infecção não tratada.
? Tem alterações da visão ou antecedentes de pressão intraocular elevada, glaucoma e/ou cataratas. Se padece alguma dessas doenças, será vigilado de perto durante o uso de Dymista.
? Tem a função adrenal deteriorada. Deve ter precaução quando se mudar um tratamento sistémico de esteroides para Dymista.
? Padece doença grave do fígado. Aumenta o risco de apresentar efeitos adversos sistémicos.
Nestes casos, o seu médico decidirá se pode utilizar Dymista.
É importante que tome a dose indicada na secção 3 ou a indicada pelo seu médico. O tratamento com doses maiores do que as recomendadas de corticoides nasais pode levar a uma supressão adrenal, o que pode produzir perda de peso, fadiga, fraqueza muscular, níveis baixos de glicose no sangue, necessidade de sal, dor nas articulações, depressão e escurecimento da pele. Se ocorrer algum desses efeitos adversos, o seu médico pode recomendar outro medicamento durante períodos de stresse ou cirurgia seletiva.
Para evitar a supressão adrenal, o seu médico aconselhará que tome a dose mais baixa que mantenha um controlo eficaz dos sintomas da rinite.
O uso de corticosteroides nasais (como Dymista) pode causar um crescimento mais lento em crianças e adolescentes quando o usam durante um longo período de tempo. O médico controlará regularmente o crescimento das crianças e se assegurará de que tomem a menor dose eficaz possível.
Entre em contato com o seu médico se experimentar visão borrada ou outras alterações visuais.
Se não tiver certeza se se encontra em alguma das situações acima, consulte o seu médico ou farmacêutico antes de usar Dymista.
Crianças
Não se recomenda o uso deste medicamento em crianças menores de 12 anos.
Uso de Dymista com outros medicamentos
Informa o seu médico ou farmacêutico se está a tomar, tomou recentemente ou pode ter que tomar qualquer outro medicamento, mesmo os adquiridos sem receita.
Alguns medicamentos podem aumentar os efeitos de Dymista, por isso o seu médico fará controles minuciosos se estiver a tomar esses medicamentos (incluídos alguns para o VIH: ritonavir, cobicistat e medicamentos para o tratamento de infecções por fungos: cetoconazol).
Gravidez e amamentação
Se estiver grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de engravidar, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Condução e uso de máquinas
Dymista exerce uma influência mínima sobre a capacidade para conduzir e usar máquinas.
Muito raramente, pode experimentar fadiga ou tontura devido à própria doença ou durante o uso de Dymista. Nesses casos, não conduza nem opere máquinas. Tenha em conta que o consumo de álcool pode potenciar esses efeitos.
Dymista contém cloreto de benzalconio.
Este medicamento contém 14 microgramas de cloreto de benzalconio em cada pulverização.
Este medicamento pode produzir irritação ou inflamação da mucosa nasal, especialmente com tratamentos de longa duração, porque contém cloreto de benzalconio. Se se suspeitar tal reação (congestão nasal persistente), sempre que seja possível, deve-se utilizar um medicamento de uso nasal que não contenha este excipiente.
3. Como usar Dymista
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
É essencial que o uso de Dymista seja regular para que o benefício terapêutico seja completo.
Evite o contacto com os olhos.
Adultos e adolescentes (maiores de 12 anos)
? A dose recomendada é de uma aplicação em cada fossa nasal, pela manhã e à noite.
Uso em crianças menores de 12 anos
?Não se recomenda o uso deste medicamento em crianças menores de 12 anos.
Uso em caso de insuficiência renale hepática
Não há dados disponíveis em pacientes com insuficiência renal e hepática.
Forma de administração
Via nasal.
Leia detenidamente as seguintes instruções e utilize o produto apenas como está indicado.
INSTRUÇÕES DE USO
Preparação da pulverização
- Agite o frasco suavemente durante 5 segundos inclinando-o de cima para baixo e, seguidamente, retire a tampa de proteção (ver figura 1).
Figura 1

- A primeira vez que se usa o pulverizador nasal, deve-se ativar a bomba pulverizando ao ar.
- Ative a bomba colocando dois dedos de cada lado da bomba de pulverização e pondo o polegar na base do frasco.
- Pulse e libere a bomba 6 vezes, até que apareça uma pulverização fina (ver figura 2).
- Agora, a bomba está ativada e pronta para ser usada.
Figura 2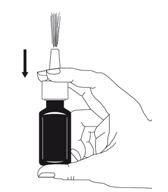
- Se não se usou o pulverizador nasal durante mais de 7 dias, terá que reativar a bomba, pressionando e liberando a mesma.
Método de pulverização
- Agite o frasco suavemente durante 5 segundos inclinando-o de cima para baixo e, seguidamente, retire a tampa de proteção (ver figura 1).
- Assoe o nariz para desobstruir as fossas nasais.
- Mantenha a cabeça inclinada para os pés. Não incline a cabeça para trás.
- Mantenha o frasco em posição reta e insira com cuidado a ponta do pulverizador em uma fossa nasal.5. Tape com o dedo a outra fossa nasal, pulse rapidamente uma vez e, ao mesmo tempo, faça uma suave inspiração (ver figura 3).
- Respire pela boca.
Figura 3

- Repita a operação na outra fossa nasal.
- Respire suavemente e não incline a cabeça para trás após a aplicação. Assim, impede-se que o medicamento chegue à garganta, provocando um sabor desagradável (ver figura 4).
Figura 4

- Depois de cada uso, limpe a ponta do pulverizador com um lenço ou pano limpo, e volte a colocar a tampa de proteção.
- Não perfure a boquilla em caso de que não se obtenha a pulverização. Limpe a válvula com água.
É importante que utilize a dose prescrita pelo seu médico. Use apenas a quantidade que lhe foi recomendada pelo seu médico.
Duração do tratamento
Dymista é apropriado para o uso prolongado. A duração do tratamento corresponde ao período em que experimenta sintomas alérgicos.
Se usar mais Dymista do que deve
Se aplicar mais quantidade deste medicamento no nariz, é improvável que sofra algum tipo de problema. Em caso de dúvida, ou se usou uma dose maior do que a recomendada durante um longo período de tempo, consulte o seu médico. Se uma pessoa, sobretudo uma criança, beber acidentalmente Dymista, consulte imediatamente o seu médico ou acuda ao centro médico mais próximo, ou ligue para o Serviço de Informação Toxicológica. Telefone (91) 562 04 20, indicando o medicamento e a quantidade ingerida.
Se esquecer de usar Dymista
Use o pulverizador nasal assim que se lembrar e, seguidamente, administre a dose seguinte à hora habitual. Não utilize uma dose dupla para compensar as doses esquecidas.
Se interromper o tratamento com Dymista
Não interrompa o tratamento sem consultar o seu médico, porque põe em risco o sucesso do seu tratamento.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Como todos os medicamentos, Dymista pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Efeitos adversos muito frequentes (podem afetar mais de 1 de cada 10 pessoas):
- Hemorragia nasal.
Efeitos adversos frequentes (podem afetar até 1 de cada 10 pessoas):
- Dor de cabeça.
- Sabor amargo na boca, especialmente se inclinar a cabeça para trás durante o uso do pulverizador nasal. Este sabor deve desaparecer se beber um refresco alguns minutos após usar este medicamento.
- Cheiro desagradável.
Efeitos adversos pouco frequentes (podem afetar até 1 de cada 100 pessoas):
- Leve irritação do interior do nariz, que pode causar leve coceira, picazón ou espirros.
- Secura nasal, tosse, secura de garganta ou irritação de garganta.
Efeitos adversos raros (podem afetar até 1 de cada 1.000 pessoas):
- Secura da boca.
Efeitos adversos muito raros (podem afetar até 1 de cada 10.000 pessoas):
- Tontura ou sonolência.
- Cataratas, glaucoma ou aumento da pressão no olho, com possível perda de visão e/ou vermelhidão e dor nos olhos. Estes efeitos adversos foram observados com tratamentos prolongados com pulverizações nasais de propionato de fluticasona.
- Lesões da pele e mucosa do nariz.
- Sensação de mal-estar, cansaço, fraqueza ou debilidade.
- Exantema, vermelhidão ou coceira da pele, urticária.
- Broncoespasmo (estreitamento das vias aéreas dos pulmões).
Solicite imediatamente atenção médica se desenvolver algum dos seguintes sintomas:
- Inchaço do rosto, lábios, língua ou garganta, que possam dificultar a deglutição/respiração e súbita aparência de exantema na pele.Estes podem ser sinais de uma reação alérgica grave. Tenha em conta que isto é muito raro.
Efeitos adversos com frequência não conhecida (a frequência não pode ser estimada a partir dos dados disponíveis)
- Visão borrada
- Úlceras no nariz
Quando este medicamento é administrado em doses elevadas durante um período prolongado de tempo, podem produzir-se efeitos adversos sistémicos (efeitos adversos que afetam todo o organismo). A probabilidade de que se produzam estes efeitos é muito menor se se utiliza uma pulverização nasal de corticosteroides em vez de corticosteroides por via oral. Estes efeitos podem variar entre pacientes individuais e entre as diferentes preparações de corticosteroides (ver secção 2).
Os corticosteroides nasais podem afetar a produção normal de hormonas no seu corpo, especialmente se forem usados altas doses durante muito tempo. Em crianças e adolescentes, este efeito adverso pode provocar um crescimento mais lento.
Em casos raros, produz-se uma redução da densidade óssea (osteoporose) quando os corticosteroides são administrados por via nasal durante um longo período de tempo.
Comunicação de efeitos adversos:
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Dymista
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no frasco e envase após “CAD”. A data de validade é o último dia do mês que se indica.
Não refrigerar ou congelar.
Validade após a abertura: Elimine qualquer resto não utilizado do medicamento 6 meses após abrir pela primeira vez o pulverizador nasal.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Desta forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e Informação adicional.
Composição de Dymista
Os princípios ativos são: hidrocloruro de azelastina e propionato de fluticasona.Cada g de suspensão contém 1.000 microgramas de hidrocloruro de azelastina e 365 microgramas de propionato de fluticasona.
Cada aplicação (0,14 g) libera 137 microgramas de hidrocloruro de azelastina (=125 microgramas de azelastina) e 50 microgramas de propionato de fluticasona.
Os demais componentes são: edetato de disódio, glicerol, celulosa microcristalina, carmelosa de sódio, polissorbato 80, cloruro de benzalconio, álcool feniletílico e água purificada.
Aspecto do produto e conteúdo do envase
Dymista é uma suspensão homogênea de cor branca.
Dymista é apresentado em um frasco de vidro de cor âmbar, equipado com uma bomba pulverizadora, um aplicador e um tampão de proteção.
O frasco de 10 ml contém 6,4 g de suspensão para pulverização nasal (pelo menos 28 aplicações). O frasco de 25 ml contém 23 g da suspensão para pulverização nasal (pelo menos 120 aplicações).
Dymista tem as seguintes apresentações:
Envase com um frasco com 6,4 g de suspensão para pulverização nasal.
Envase com um frasco com 23 g de suspensão para pulverização nasal.
Envase múltiplo que contém 10 frascos com 6,4 g, cada um, de suspensão para pulverização nasal
Envase múltiplo que contém 3 frascos com 23 g, cada um, de suspensão para pulverização nasal
Nem todos os tamanhos de envase podem estar comercializados.
Titular da Autorização de Comercialização
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Dublín 15
Dublín
Irlanda
Responsável pela fabricação
Mylan Hungary Kft,
H-2900 Komárom,
Mylan utca 1,
Hungria
ou
MEDA Pharma GmbH & Co. KG
Benzstrasse 1
61352 Bad Homburg
(Alemanha)
ou
Haupt Pharma Amareg GmbH
Donaustaufer Str. 378
93055 Regensburg
(Alemanha)
ou
Madaus GmbH
Lütticher Straße 5
53842 Troisdorf
Alemanha
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do titular da autorização de comercialização:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 - Madrid
Espanha
Este medicamento está autorizado nos estados membros do Espaço Económico Europeue no Reino Unido (Irlanda do Norte)com os seguintes nomes:
Áustria Dymista Nasenspray
Bulgária Dymista
Chipre Dymista Ρινικ? εκν?φωμα
República Checa Dymistin 137 mikrogramu / 50 mikrogramu, nosní sprej, suspenze
Dinamarca Dymista
Estônia Dymista
Finlândia Dymista nenäsumute
França Dymistalin Suspension pour pulvérisation nasale
Alemanha Dymista Nasenspray 137 Mikrogramm/50 Mikrogramm pro SprühstoßNasenspray, Suspension
Grécia Dymista Ρινικ? εκν?φωμα
Hungria Dymista Szuszpenziós orrspray
Islândia Dymista Nefúði
Irlanda Dymista Nasal Spray
Itália Dymista
Letônia Dymista 137 mikrogrami/50 mikrogrami deva deguna aerosols, suspensija
Lietchenstein Dymista Nasenspray
Lituânia Dymista 137 mikrogramai/50 mikrogramu / dozeje nosies purškalas
Luxemburgo Dymista Neusspray / Suspension pour pulvérisation nasale / Nasenspray
Malta Dymista Nasal Spray
Noruega Dymista nesespray
Polônia Dymista
Portugal Dymista Spray nasal
Romênia Dymista 137 micrograme / 50 micrograme /doza spray nazal suspensie
Eslováquia Dymista nosová aerodisperzia
Eslovênia Dymista 137 mikrogramov / 50 mikrogramov na vpih pršilo za nos, suspenzija
Espanha Dymista suspensão pulverização nasal
Suécia Dymista Nässpray, suspension (1mg/g; 0.365 mg/g)
Reino Unido (Irlanda do Norte) Dymista Nasal Spray
Data da última revisão deste prospectofevereiro 2019
A informação detalhada e atualizada sobre este medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS)https://www.aemps.gob.es

Quanto custa o DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL em Espanha em 2025?
O preço médio do DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL em dezembro de 2025 é de cerca de 15.61 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia15.61 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASALForma farmacêutica: PRODUTO PARA USO NASAL, 137 microgramas/50 microgramas/aplicaçãoSubstância ativa: fluticasone, combinationsFabricante: Laboratorios Cinfa S.A.Requer receita médicaForma farmacêutica: PRODUTO PARA USO NASAL, 137 microgramas/50 microgramas/ativaçãoSubstância ativa: fluticasone, combinationsFabricante: Teva B.V.Requer receita médicaForma farmacêutica: PRODUTO PARA USO NASAL, 137 microgramas/50 microgramas/aplicaçãoSubstância ativa: fluticasone, combinationsFabricante: Especialidades Farmaceuticas Centrum S.A.Requer receita médica
Alternativas a DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL em Poland
Alternativa a DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL em Ukraine
Médicos online para DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de DYMISTA 137 microgramas/50 microgramas/aplicação SUSPENSÃO PARA PULVERIZAÇÃO NASAL – sujeita a avaliação médica e regras locais.








