
CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO

Pergunte a um médico sobre a prescrição de CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO

Como usar CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO
Introdução
Prospecto: informação para o paciente
Ceftazidima Qilu 1 g pó para solução injetável e para perfusão EFG
ceftazidima
Leia todo o prospecto detenidamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou enfermeiro.
- Se experimentar efeitos adversos, consulte o seu médico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Ceftazidima Qilu e para que é utilizado
- O que precisa saber antes de começar a usar Ceftazidima Qilu
- Como usar Ceftazidima Qilu
- Efeitos adversos possíveis
- Conservação de Ceftazidima Qilu
- Conteúdo do envase e informação adicional
1. O que é Ceftazidima Qilu e para que é utilizado
A ceftazidima é um antibiótico que é empregue em adultos e crianças (incluindo os recém-nascidos) e que actua matando as bactérias que causam infecções. Pertence a um grupo de medicamentos denominados cefalosporinas.
Os antibióticos são utilizados para tratar infecções bacterianas e não servem para tratar infecções virais como a gripe ou o resfriado.
É importante que siga as instruções relativas à dose, ao intervalo de administração e à duração do tratamento indicadas pelo seu médico.
Não guarde nem reutilize este medicamento. Se uma vez finalizado o tratamento lhe sobrar antibiótico, devolva-o à farmácia para a sua correta eliminação. Não deve deitar os medicamentos pelo esgoto nem para o lixo.
A ceftazidima é utilizada para tratar infecções bacterianas graves de:
- os pulmões ou o peito
- os pulmões e os brônquios em pacientes que padecem de fibrose quística
- o cérebro (meningite)
- os ouvidos
- as vias urinárias
- a pele e os tecidos moles
- o abdômen e a parede abdominal (peritonite)
- os ossos e as articulações
A ceftazidima também pode ser utilizada:
- para prevenir infecções durante a cirurgia de próstata nos varões.
- para tratar pacientes que apresentem um recuento baixo de glóbulos brancos (neutropenia) e febre devida a uma infecção bacteriana.
2. O que precisa saber antes de começar a usar Ceftazidima Qilu
Não use Ceftazidima:
- se é alérgico à ceftazidima ou a algum dos outros componentes deste medicamento (incluídos na seção 6).
- se padeceu uma reação alérgica grave a outro antibiótico (penicilinas, monobactâmicos e carbapenémicos), pois também poderia ser alérgico à ceftazidima.
→Se acredita que se encontra em algum destes casos, informe o seu médicoantes de que lhe administrem Ceftazidima Qilu. De ser assim, não deve receber este medicamento.
Advertências e precauções
Consulte o seu médico ou enfermeiro antes de que lhe administrem Ceftazidima Qilu.
Enquanto estiver a receber este medicamento deve estar atento a determinados sintomas, como reações alérgicas, transtornos do sistema nervoso e transtornos gastrointestinais, como diarreia. Com isso se reduzirá o risco de possíveis problemas. Consulte o apartado Doenças que requerem uma atenção especialda seção 4. Se padeceu uma reação alérgica a outros antibióticos, também poderia ser alérgico à ceftazidima.
Foram notificadas reações cutâneas graves, incluindo síndrome de Stevens-Johnson, necrólise epidérmica tóxica, reação a fármaco com eosinofilia e sintomas sistémicos (RFESS) e pustulose exantemática generalizada aguda (PEGA) em relação com o tratamento com ceftazidima. Procure atenção médica de imediato se notar algum dos sintomas relacionados com estas reações cutâneas graves descritos na seção 4.
Se precisa de uma análise de sangue ou urina
A ceftazidima pode afectar os resultados das análises de glicose (açúcar) na urina e a uma análise de sangue que se denomina prova de Coombs. Se vão fazer-lhe análises:
→Informe a pessoa que lhe extrai a amostrade que lhe foram administrados Ceftazidima Qilu.
Uso de Ceftazidima Qilu com outros medicamentos
Informe o seu médico se está a tomar, tomou recentemente ou poderia ter que tomar qualquer outro medicamento,o que inclui os medicamentos que pode comprar sem receita médica.
Não devem administrar-lhe ceftazidima sem consultá-lo primeiro ao seu médico se também está a tomar:
- Um antibiótico denominado cloranfenicol.
- Um tipo de antibiótico denominado aminoglicósido(p. ex., gentamicina ou tobramicina).
- Diuréticos denominados furosemida
→Se encontra-se em alguma destas situações, informe o seu médico.
Gravidez e amamentação
Se está grávida ou em período de amamentação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico antes de utilizar este medicamento.
O seu médico avaliará o benefício de o tratar com ceftazidima face ao risco para o seu filho.
Condução e uso de máquinas
Este medicamento pode causar efeitos adversos que afectam a sua capacidade para conduzir, como tontura. Não conduza nem utilize máquinas, a não ser que esteja seguro de que isso não o afecta.
Ceftazidima Qilu contém sódio.
Deve ter isto em conta se segue uma dieta baixa em sódio.
Ceftazidima Qilu (concentração) | Quantidade por frasco |
Ceftazidima Qilu 1 g | Este medicamento contém 52 mg de sódio (componente principal do sal de mesa e de cozinha) em cada frasco, o que equivale a 2,6 % da ingestão diária máxima recomendada de sódio nas refeições para um adulto. |
3. Como usar Ceftazidima Qilu
CeftazidimaQilu é geralmente administrado por um médico ou um enfermeiro.
Ceftazidima Qilu 1 g pode ser administrado por injeção ou perfusão intravenosa ou injeção intramuscular profunda.
O médico, o enfermeiro ou o farmacêutico prepararão Ceftazidima Qilu com água para preparações injetáveis ou um líquido para perfusão adequado.
A dose recomendada é:
A dose correcta de ceftazidima que lhe será administrada será decidida pelo seu médico e depende da gravidade e do tipo de infecção, se está a tomar outros antibióticos, o seu peso e idade e o quão bem estão a funcionar os seus rins.
Recém-nascidos (0-2meses)
Por cada 1kg de peso que pese o bebê, lhe serão administrados 25-60 mg de ceftazidima por dia, divididos em duas doses.
Lactentes (com mais de 2meses) e criançasque pesem menos de 40 kg
Por cada 1kg de peso que pese o bebê ou a criança, lhe serão administrados 100-150 mg de ceftazidima por dia, divididos em três doses. A dose máxima é de 6 g por dia.
Adultos e adolescentescom um peso de 40 kg ou mais
De 1 g a 2 g de ceftazidima três vezes por dia. A dose máxima é de 9 g por dia.
Pacientes com mais de 65anos de idade
A dose diária não deve ultrapassar, por regra geral, os 3 g por dia, especialmente se tiver mais de 80 anos de idade.
Pacientes com problemas de rim
É possível que lhe sejam administradas doses diferentes das habituais. O médico ou o enfermeiro decidirão a quantidade de Ceftazidima Qilu que precisa, com base na gravidade da doença renal que padece. O seu médico o vigiará de perto, e é possível que lhe sejam realizadas análises da actividade renal com mais frequência.
Se usar mais Ceftazidima Qilu do que deve
Se considera que acidentalmente lhe foram administrados mais medicamentos do que a dose que lhe foi prescrita, contacte o seu médico ou o hospital mais próximo de imediato.
Em caso de sobredose ou ingestão acidental, consulte imediatamente o seu médico ou farmacêutico ou ligue para o Serviço de Informação Toxicológica, telefone: 91 562 04 20, indicando o medicamento e a quantidade ingerida.
Se esquecer de usar Ceftazidima Qilu
Se saltar uma injeção deste medicamento, deve recebê-la o mais breve possível. Não devem administrar-lhe uma dose dupla (duas injeções ao mesmo tempo) para compensar a dose omitida.
Se interromper o tratamento com Ceftazidima Qilu
Não deixe de receber Ceftazidima Qilu a menos que o seu médico o indique.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou enfermeiro.
4. Efeitos adversos possíveis
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Doenças que requerem uma atenção especial
Os seguintes efeitos secundários graves foram notificados em um número reduzido de pessoas (a frequência exacta é desconhecida):
- reação alérgica grave. Os sinais incluem erupção abultada e com picaz, inchação, algumas vezes na face ou na boca que causa dificuldade para respirar.
- Erupção cutâneaque pode formar bolhase aparece como pequenas dianas(ponto central escuro, rodeado de uma área mais pálida, com um anel escuro ao redor do bordo).
- Uma erupção generalizadaacompanhada de bolhase descamação da pele, úlceras na boca, na garganta, no nariz, nos genitais ou nos olhos. Estas erupções cutâneas graves podem ser precedidas de febre e sintomas pseudogripais (Estes sinais podem corresponder ao síndrome de Stevens-Johnsonou à necrólise epidérmica tóxica).
- Transtornos do sistema nervoso: tremores, ataques e, em alguns casos, coma. Estes efeitos adversos ocorreram em pacientes a quem foi administrada uma dose excessiva, especialmente em pacientes com doença renal.
- Foram notificados casos raros de reações de hipersensibilidade graves, acompanhadas de erupção grave, que poderia ir acompanhada de febre, fadiga, inchação da face ou dos gânglios linfáticos, aumento de eosinófilos (um tipo de glóbulo branco), efeitos no fígado, nos rins ou nos pulmões (uma reação denominada DRESS).
- Erupção disseminada e avermelhada com descamação, protuberâncias sob a pele e bolhas acompanhada de febre. Os sintomas geralmente aparecem no início do tratamento (pustulose exantemática generalizada aguda).
Outros efeitos adversos:
Frequentes: podem afectar até 1 de cada 10 pessoas
- diarreia
- inchação e avermelhamento ao redor de uma veia
- erupção cutânea vermelha e abultada, que pode produzir picaz
- dor, queimadura, inchação ou inflamação no local de injeção
- Se lhe preocupa algum destes sintomas, informe o seu médico.
Os efeitos adversos frequentes que podem aparecer nas análises de sangue são:
- um aumento do recuento de um tipo de glóbulo branco (eosinofilia)
- um aumento do recuento das células que intervêm na coagulação do sangue
- um aumento das enzimas do fígado
Pouco frequentes
- inflamação do intestino, que pode causar dor, ou diarreia que pode conter sangue
- candidíase (infecções fúngicas na boca ou na vagina)
- cefaleia
- tontura
- dor de estômago
- sensação de mal-estar ou tontura
- febre e calafrios.
- Se padece algum destes sintomas, informe o seu médico.
Os efeitos adversos pouco frequentes que podem aparecer nas análises de sangue são:
- uma diminuição do recuento de glóbulos brancos.
- uma diminuição do recuento de plaquetas (as células que ajudam na coagulação do sangue)
- um aumento da concentração de ureia, o nitrogénio ureico ou a creatinina sérica no sangue
Muito raros
- inflamação ou insuficiência dos rins
Frequência não conhecida: não pode ser estimada a partir dos dados disponíveis.
- inflamação ou insuficiência dos rins
- formigamento
- mau sabor de boca
- coloração amarelada do branco dos olhos ou da pele
Os efeitos adversos de frequência não conhecida que podem aparecer nas análises de sangue são:
- destruição demasiado rápida dos glóbulos vermelhos
- um aumento do recuento de um tipo determinado de glóbulos brancos
- uma diminuição aguda do recuento de glóbulos brancos
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano www.notificaram.es.
Ao comunicar efeitos adversos, você pode contribuir para proporcionar mais informações sobre a segurança deste medicamento.
5. Conservação de Ceftazidima Qilu
Mantenha este medicamento fora da vista e do alcance das crianças.
- Não utilize este medicamento após a data de validade que aparece no frasco e na caixa após CAD. A data de validade é o último dia do mês que se indica.
- Conservar abaixo de 25 °C.
- Conservar os frascos no embalagem exterior para protegê-los da luz.
- Solução reconstituída e diluída: o médico, o farmacêutico ou o enfermeiro prepararão o seu medicamento com água para preparações injetáveis ou líquidos compatíveis.
- A estabilidade física e química durante o uso é de 24 horas a uma temperatura de entre 2 ºC e 8 ºC e de 2 horas a 25 ºC.
- Desde um ponto de vista microbiológico, o fármaco deve ser utilizado imediatamente, a menos que o método de abertura, reconstituição e diluição evite o risco de contaminação microbiológica. Se não for utilizado imediatamente, o tempo e as condições de conservação durante o uso são responsabilidade do utilizador.
- Os medicamentos não devem ser deitados pelo esgoto nem para o lixo. Deposite os envases e os medicamentos que não precisa no ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Deste modo, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Ceftazidima Qilu
Ceftazidima Qilu está disponível nas apresentações seguintes: 2 g, 1 g e 500 mg.
- O princípio ativo é 2 g, 1 g ou 500 mg de ceftazidima (na forma de ceftazidima pentahidrato).
- Os outros componentes são: carbonato de sódio.
Consulte a seção 2 referente à informação importante sobre o sódio, um dos componentes de Ceftazidima Qilu.
Aspecto do produto e conteúdo do envase
Ceftazidima Qilu 1 g pó para solução injetável e para perfusão é um pó estéril cristalino de cor branca ou esbranquiçada, contido em um frasco de vidro de 15 ml de capacidade, com um tampão de borracha de butilo e selo de alumínio.
Disponível em envases de 1, 10 ou 50 frascos.
Pode ser que apenas alguns tamanhos de envases estejam comercializados.
O seu médico, farmacêutico ou enfermeiro prepararão a injeção ou a perfusão com água para preparações injetáveis ou um líquido para perfusão adequado. Ceftazidima Qilu varia de cor quando é reconstituído, de amarelo claro a cor âmbar. Isto é completamente normal.
Titular da autorização de comercialização e responsável pela fabricação
QILU PHARMA SPAIN S.L.,
Paseo de la Castellana 40
planta 8, 28046 - Madrid
Espanha
Representante local
Sun Pharma Laboratórios, S.L.
Rambla de Catalunya 53-55
08007 – Barcelona, Espanha
Responsável pela fabricação
KYMOS, S.L
Ronda de Can Fatjó,
7B (Parque Tecnológico del Vallès),
Cerdanyola del Vallès, 08290,
Barcelona, Espanha
NETPHARMALAB CONSULTING SERVICES
Carretera de Fuencarral 22
Alcobendas, 28108 - Madrid,
Espanha
MIAS Pharma Limited
Suite 2, Stafford House, Strand Road, Portmarnock, Co. Dublin,
Irlanda
Tillomed Malta Ltd.
Malta Life Sciences Park, LS2.01.06 Industrial Estate, San Gwann, SGN 3000,
Malta
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Reino Unido | Ceftazidima 1 g pó para solução para injeção/infusão |
Alemanha | Ceftazidim Qilu 1 g pó para solução injetável/infusão |
Espanha | Ceftazidima Qilu 1 g pó para solução injetável e para perfusão EFG |
França | CEFTAZIDIME QILU 1 g, pó para solução injetável/para perfusão (IM, IV) |
Itália | Ceftazidima Qilu |
Suécia | Ceftazidim Qilu 1 g pó para solução injetável/infusão, solução |
Data da última revisão deste folheto: 10/2024.
A informação detalhada e atualizada deste medicamento está disponível no site da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/.
------------------------------------------------------------------------------------------------------------------------
Esta informação está destinada apenas a profissionais do setor sanitário:
Ceftazidima Qilu 1 g pó para solução injetável e para perfusão EFG
Ceftazidima
Por favor, consulte a informação detalhada da Ficha técnica ou resumo das características do produto.
Após a reconstituição:
A estabilidade física e química durante o uso é de 24 horas a uma temperatura de entre 2 °C e 8 °C e de 2 horas a 25 °C em água para preparações injetáveis ou os líquidos compatíveis que se enumeram a seguir.
Do ponto de vista microbiológico, o fármaco deve ser utilizado imediatamente, a menos que o método de abertura, reconstituição e diluição evite o risco de contaminação microbiológica.
Se não for utilizado imediatamente, o tempo e as condições de conservação durante o uso são responsabilidade do usuário.
Após a diluição:
A estabilidade física e química durante o uso é de 24 horas a uma temperatura de entre 2 °C e 8 °C e de 2 horas a 25 °C em água para preparações injetáveis ou os líquidos compatíveis que se enumeram a seguir.
Do ponto de vista microbiológico, o fármaco deve ser utilizado imediatamente, a menos que o método de abertura, reconstituição e diluição evite o risco de contaminação microbiológica.
Se não for utilizado imediatamente, o tempo e as condições de conservação durante o uso são responsabilidade do usuário.
Precauções especiais de conservação
Conservar abaixo de 25 °C.
Conservar os frascos no embalagem exterior para protegê-los da luz.
Precauções especiais de eliminação e outras manipulações
À medida que o produto se dissolve, é liberado dióxido de carbono e se desenvolve uma pressão positiva. Devem ser ignoradas as pequenas bolhas de dióxido de carbono na solução reconstituída.
Instruções para a reconstituição
Consulte os volumes de adição e as concentrações da solução das Tabelas 1 e 2 que podem ser úteis quando se necessitam doses fracionadas.
Tabela 1. Pó para solução injetável
Apresentação | Quantidade de diluente que se tem que adicionar (ml) | Concentração aproximada (mg/ml) | |
1 g | |||
Intramuscular | 3 ml | 260 | |
Bolo intravenoso | 10 ml | 90 |
Nota:
- O volume resultante da solução de ceftazidima no meio reconstituído aumenta como consequência do fator de deslocamento do fármaco resultante nas concentrações enumeradas em mg/ml que se apresentam na tabela anterior.
Tabela 2. Pó para solução para perfusão
Apresentação | Quantidade de diluente que se tem que adicionar (ml) | Concentração aproximada (mg/ml) | |
1 g | |||
Infusão intravenosa | 50 ml* | 20 |
- A adição deve ser efetuada em duas etapas.
Nota:
- O volume resultante da solução de ceftazidima no meio reconstituído aumenta como consequência do fator de deslocamento do fármaco resultante nas concentrações enumeradas em mg/ml que se apresentam na tabela anterior.
A cor das soluções varia de amarelo pálido a âmbar, em função da concentração, dos diluentes e das condições de conservação empregadas. A potência do produto não é afetada negativamente por tais variações da cor, dentro das recomendações indicadas.
A ceftazidima em concentrações compreendidas entre 1 mg/ml e 40 mg/ml é compatível com:
- Solução injetável de cloreto de sódio a 0,9 % (9 mg/ml)
- Solução injetável de lactato de sódio M/6
- Solução injetável de lactato de sódio composta (solução de Hartmann)
- Solução injetável de glicose a 5 % (50 mg/ml)
- Solução injetável de cloreto de sódio a 0,225 % (2,25 mg/ml) e glicose a 5 % (50 mg/ml)
- Solução injetável de cloreto de sódio a 0,45 % (4,5 mg/ml) e glicose a 5 % (50 mg/ml)
- Solução injetável de cloreto de sódio a 0,9 % (9 mg/ml) e glicose a 5 % (50 mg/ml)
- Solução injetável de cloreto de sódio a 0,18 % (1,8 mg/ml) e glicose a 4 % (40 mg/ml)
- Solução injetável de glicose a 10 % (100 mg/ml)
- Solução injetável de dextrano 40 a 10 % (100 mg/ml) em solução injetável de cloreto sódico a 0,9 % (9 mg/ml)
- Solução injetável de dextrano 40 a 10 % (100 mg/ml) em solução injetável de glicose a 5 % (50 mg/ml)
- Solução injetável de dextrano 70 a 6 % (60 mg/ml) em solução injetável de cloreto sódico a 0,9 % (9 mg/ml)
- Solução injetável de dextrano 70 a 6 % (60 mg/ml) em solução injetável de glicose a 5 % (50 mg/ml)
A ceftazidima em concentrações compreendidas entre 0,05 mg/ml (0,005 %) e 0,25 mg/ml (0,025 %) é compatível com o líquido de diálise intraperitoneal (lactato).
A ceftazidima pode ser reconstituída para uso intramuscular com solução injetável de hidrocloruro de lidocaína a 0,5 % (5 mg/ml) ou a 1 % (10 mg/ml) com as concentrações detalhadas na Tabela 1.
O conteúdo de um frasco de 500 mg de Ceftazidima Qilu, reconstituído com 1,5 ml de água para preparações injetáveis, pode ser adicionado a uma injeção de metronidazol (500 mg em 100 ml), em que ambos conservarão sua atividade.
500 mg pó para solução injetável, 1 g, 2 g pó para solução injetável ou para perfusão:
ESTE APARTADO SE DEVE APRESENTAR COM PICTOGRAMAS, AO MESMO TEMPO QUE NO MATERIAL ANTERIOR.
Preparação de soluções para injeção em bolo:
- Insira a agulha da seringa através do fecho do frasco e injete o volume recomendado de diluente. Retire a agulha da seringa.
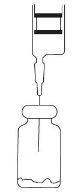
- Agite até que se dissolva: é liberado o dióxido de carbono e se obtém uma solução transparente em 1 ou 2 minutos.
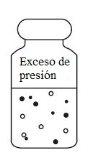
- Vire o frasco. Com o êmbolo da seringa totalmente comprimido, insira a agulha através do fecho do frasco e extraia na seringa o volume total da solução (a pressão criada no frasco pode ajudar na extração). Certifique-se de que a agulha permaneça dentro da solução e não no espaço livre superior. A solução extraída pode conter pequenas bolhas de dióxido de carbono que podem ser ignoradas.
Estas soluções de ceftazidima podem ser administradas diretamente na veia ou introduzidas na sonda de um equipamento de nutrição enteral no caso de que o paciente esteja recebendo líquidos por via parenteral. A ceftazidima é compatível com os líquidos intravenosos enumerados anteriormente.
1 g, 2 g pó para solução injetável e para perfusão:
Preparação de soluções para perfusão i.v. de Ceftazidima Qilu em apresentações habituais em frasco (minibolsa ou sistema de perfusão de tipo bureta):
Prepare com um total de 50 ml (para frascos de 1 g e 2 g) de diluente compatível (dos enumerados acima), adicionado em DUAS etapas como se explica a seguir.
- Insira a agulha da seringa através do fecho do frasco e injete 10 ml de diluente para os frascos de 1 g e 2 g.
- Retire a agulha e agite o frasco até obter uma solução transparente.
- Não insira uma agulha para liberar o gás até que não se tenha dissolvido o produto. Insira uma agulha para liberar o gás através do fecho do frasco para liberar a pressão interna.
- Transfira a solução reconstituída para o veículo final de distribuição (p. ex., minibolsa ou sistema de perfusão de tipo bureta) até atingir um volume total de pelo menos 50 ml e administre mediante perfusão intravenosa durante 15-30 minutos.
Nota: Para conservar a esterilidade do produto é importante que a agulha para liberar o gás não seja inserida através do fecho do frasco até que não se tenha dissolvido o produto.
Deve-se descartar toda solução de antibiótico sobrante.
Para um único uso.
A eliminação do medicamento não utilizado e de todos os materiais que tenham estado em contato com ele será realizada de acordo com a normativa local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃOForma farmacêutica: INJETÁVEL, 1 gSubstância ativa: ceftazidimeFabricante: Fresenius Kabi España, S.A.U.Requer receita médicaForma farmacêutica: INJETÁVEL, 2000 mgSubstância ativa: ceftazidimeFabricante: Fresenius Kabi España, S.A.U.Requer receita médicaForma farmacêutica: INJETÁVEL, 1 gSubstância ativa: ceftazidimeFabricante: Ldp Laboratorios Torlan S.A.Requer receita médica
Alternativas a CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO em Polónia
Alternativa a CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO em Ukraine
Médicos online para CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de CEFTAZIDIMA QILU 1 g PÓ PARA SOLUÇÃO INJETÁVEL E PARA PERFUSÃO – sujeita a avaliação médica e regras locais.














