LEUPRORELIN GP-PHARM DEPOT TRIMESTRAL 22.5 mg POWDER AND SOLVENT FOR PROLONGED RELEASE INJECTABLE SUSPENSION

How to use LEUPRORELIN GP-PHARM DEPOT TRIMESTRAL 22.5 mg POWDER AND SOLVENT FOR PROLONGED RELEASE INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Leuprorelin GP-Pharm Depot Trimestral is and what it is used for
- What you need to know before you use Leuprorelin GP-Pharm Depot Trimestral
- How to use Leuprorelin GP-Pharm Depot Trimestral
- Possible side effects
- Storage of Leuprorelin GP-Pharm Depot Trimestral
- Container Contents and Additional Information
Introduction
Package Leaflet: Information for the User
Leuprorelin GP-Pharm Depot Trimestral 22.5 mg powder and solvent for prolonged-release suspension for injection
Leuprorelin acetate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Leuprorelin GP-Pharm Depot Trimestral is and what it is used for
- What you need to know before you use Leuprorelin GP-Pharm Depot Trimestral
- How to use Leuprorelin GP-Pharm Depot Trimestral
- Possible side effects
- Storage of Leuprorelin GP-Pharm Depot Trimestral
- Contents of the pack and other information
1. What Leuprorelin GP-Pharm Depot Trimestral is and what it is used for
Leuprorelin GP-Pharm Depot Trimestral is a vial containing a white powder, which is reconstituted as a suspension for injection into a muscle. This medicine contains the active substance leuprorelin (also known as leuprolide), which belongs to a group of medicines called gonadotropin-releasing hormone (LHRH) agonists, which are medicines that reduce testosterone (a sex hormone).
Your doctor has prescribed this medicine for the palliative treatment of advanced prostate cancer.
2. What you need to know before you use Leuprorelin GP-Pharm Depot Trimestral
Do not use Leuprorelin GP-Pharm Depot Trimestral:
- If you are allergic (hypersensitive) to LHRH, LHRH agonists or any of the other ingredients of this medicine (listed in section 6). An allergic reaction may appear as a skin rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue.
- If you have undergone orchiectomy (removal of the testicles).
- If you are a woman or a child.
- This medicine must not be used alone (as monotherapy) for the treatment of prostate cancer when the spinal cord is compressed or the cancer has spread to the spinal cord.
Warnings and precautions
- Talk to your doctor or pharmacist before starting this medicine.
- It is possible that your disease may worsen during the first few weeks of treatment, but it should improve with continued treatment. The signs and symptoms include: temporary increase in testosterone (male hormone), hot flashes, bone pain, nervous system disorders (including depression) or urinary obstruction.
- If you think you are experiencing an allergic reaction (shortness of breath, asthma, rhinitis, swelling of the face, urticaria, skin rash), stop taking this medicine and inform your doctor.
- Tell your doctor if you have a risk of or are already suffering from any of the following diseases, as you may need more frequent check-ups:
- Bruises or unexplained bleeding or if you feel unwell. Although rare, these can be symptoms of changes in the number of red or white blood cells.
- Metabolic disease
- Heart problems or palpitations
- Diabetes.
- Your doctor should be informed of any personal medical history of pituitary adenoma (non-cancerous tumor of the pituitary gland). Cases of pituitary apoplexy (partial loss of pituitary tissue) have been described after the initial administration of this type of medicine to patients with pituitary adenoma. Pituitary apoplexy may manifest as sudden headache, meningism, vision disorders or altered vision, including blindness, and occasionally a decrease in the level of consciousness.
- Your doctor should know if you suffer from a coagulation disorder, thrombocytopenia or if you are being treated with anticoagulants. Your liver function may need to be monitored, as liver disorders and jaundice (yellowing of the eyes and skin) have been described with the administration of leuprorelin.
- Spinal fracture, paralysis, low blood pressure and high blood pressure have been described with leuprorelin treatment.
- Depression has been reported in patients undergoing treatment with this medicine, which can be severe. If you are taking this medicine and feel depressed, inform your doctor.
- A reduction in bone density (fragile or thinner bones) has been described after administration of leuprorelin. Your doctor may consider adding an anti-androgen to the treatment with this medicine. In this case, your doctor will be alert to detect the presence of vein inflammation (thrombophlebitis) and other signs of coagulation disorders and edema (swelling of hands, feet or ankles) that are more likely to occur when anti-androgenic treatment is added to this medicine.
- Tell your doctor if you feel pressure on the spinal cord and/or present urinary disorders and/or hematuria (blood in the urine); in such a case, your doctor will discuss the need for additional treatments to prevent neurological complications (e.g., tingling in hands and feet, paralysis) or urethral obstruction (the duct that connects the bladder to the outside of the body). You will be closely monitored during the first few weeks of treatment.
- Patients may experience metabolic changes (e.g., glucose intolerance or worsening of existing diabetes), weight changes, and cardiovascular disorders.
- Patients with metabolic or cardiovascular disease, and especially those with a history of congestive heart failure (a disease in which the heart can no longer pump enough blood to the rest of the body), should be monitored during treatment with leuprorelin.
- Ask your doctor, pharmacist, or nurse if you have fatty liver.
- During treatment, some blood tests will be performed to check if this medicine is effective.
- You may experience a loss of interest in sexual relations, hot flashes, and occasionally a reduction in the size and function of the testicles.
- You may become fertile again when treatment with this medicine is interrupted.
- This medicine may interfere with certain laboratory tests, so you should ensure that your doctor knows that you are taking this medicine.
- Seizures may occur in predisposed patients (patients with a history of seizures, epilepsy, cerebrovascular disorders, anomalies or tumors of the central nervous system), in patients taking drugs that may cause seizures, and to a lesser extent in patients who do not have these characteristics.
- Tell your doctor if you have any heart or blood vessel disorder or are being treated for it, including medications to control heart rhythm (arrhythmias). The risk of heart rhythm problems may increase when using this medicine.
- Contact your doctor immediately if you experience severe or recurrent headaches, vision problems, or tinnitus (ringing in the ears).
- Severe skin reactions, including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), have been reported in association with leuprorelin. Discontinue the use of leuprorelin and seek medical attention immediately if you notice any of the symptoms related to these severe skin reactions described in section 4.
Using Leuprorelin GP-Pharm Depot Trimestral with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. It may still be suitable to use this medicine; your doctor will decide what is appropriate for you.
This medicine may interfere with some medicines used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with other medicines (e.g., methadone (used for pain relief and for detoxification from other medicines), moxifloxacin (an antibiotic), antipsychotics used to treat serious mental illnesses).
Pregnancy and breastfeeding
The use of this medicine is not indicated in women.
This medicine is contraindicated during pregnancy. Spontaneous abortions may occur if this medicine is administered during pregnancy.
Driving and using machines
There are no specific studies on the effects of this medicine on the ability to drive and use machines.
Visual disturbances and dizziness may occur during treatment. If you are affected, do not drive or operate machinery.
Leuprorelin GP-Pharm Depot Trimestralcontainsless than 23 mg of sodium (1 mmol) per dose; it is essentially “sodium-free”.
3. How to use Leuprorelin GP-Pharm Depot Trimestral
Posology
This medicine should only be administered by your doctor or nurse. They will be responsible for preparing the product.
Adults and the elderly:
The recommended dose of this medicine is one injection every three months. The powder is reconstituted to form a suspension that is administered as an intramuscular injection (into a muscle) once every three months.
The injection site should be varied at regular intervals.
This medicine must be administered only by the intramuscular route. It must not be administered by any other route.
The treatment schedule will be decided by your doctor.
Use in children:This medicine is not indicated in children.
If you use more Leuprorelin GP-Pharm Depot Trimestral than you should
This is unlikely, as your doctor or nurse will know what dose is appropriate. However, if you suspect that you have received more medicine than you should, inform your doctor immediately so that the necessary measures can be taken.
In case of overdose or accidental ingestion, consult the Toxicological Information Service, tel: 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Leuprorelin GP-Pharm Depot Trimestral
It is important that you do not miss a dose of this medicine. As soon as you realize that you have missed an injection, contact your doctor, who will administer the next injection.
If you stop using Leuprorelin GP-Pharm Depot Trimestral
Since medical treatment involves the administration of this medicine for a long period, if treatment is interrupted, you may experience a worsening of the symptoms related to the disease. Therefore, do not stop treatment prematurely without your doctor's permission.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you notice any of the following symptoms:
- You suffer from wheezing, difficulty breathing, swelling of the eyelids, face or lips, skin rash or itching (especially if it affects your whole body) suddenly.
- Frequency not known (frequency cannot be estimated from the available data):
- If you notice circular or target-shaped red spots on your trunk, often with central blisters, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin reactions may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome/toxic epidermal necrolysis).
- Redness of the skin and rash with itching (toxic skin rash).
- A skin reaction that causes grains or red spots on the skin, which may look like a target, with a dark red center surrounded by lighter red rings (erythema multiforme).
The following side effects have been reported:
Very common (may affect more than 1 in 10 people):
Hot flashes and reactions at the injection site.
Common (may affect up to 1 in 10 people):
Cold sweats, hyperhidrosis (increased sweating), pruritus (itching), fatigue, insomnia, decreased libido, dizziness, flushing, nausea, diarrhea, decreased appetite, erectile dysfunction, asthenia (lack or loss of strength), bone pain, joint pain, and reactions at the injection site such as pain, irritation, erythema (redness of the skin). Urinary tract pain, decreased urine flow, frequent urination, mood changes, and depression in prolonged treatments with leuprorelin, alteration of liver enzymes, hyperlipidemia (high lipid levels in the blood), increased blood sugar.
Uncommon (may affect up to 1 in 100 people):
High cholesterol, sleep disorders, restlessness, altered taste, paresthesia (altered skin sensitivity), headache, lethargy (drowsiness), blurred vision, pleurisy, tinnitus (ringing in the ears), upper abdominal pain, constipation, papule, rash, generalized pruritus (itching), night sweats, back pain, muscle pain, neck pain, breast pain, pelvic pain, testicular atrophy, testicular disorder, sensation of heat, mood changes, and depression in short-term treatments with leuprorelin. Changes in blood values and electrocardiogram (ECG) (prolongation of the QT interval). Reactions at the injection site such as urticaria, heat, and hemorrhage.
Not known (frequency cannot be estimated from the available data)
Lung inflammation, lung disease
Idiopathic intracranial hypertension (increased intracranial pressure around the brain characterized by headaches, diplopia, and other visual symptoms, tinnitus or ringing in one or both ears).
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Leuprorelin GP-Pharm Depot Trimestral
Your doctor or pharmacist will know how to store this medicine.
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Do not freeze.
Do not use this medicine after the expiry date which is stated on the carton, vial, and syringe after “EXP”. The syringe has the same expiry date as the vial.
The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Composition of Leuprorelina GP-Pharm Depot Trimestral
The active ingredient is leuprorelin acetate. Each vial contains 22.5 mg of leuprorelin acetate.
The concentration of the reconstituted product is 11.25 mg/ml. The other components are: polysorbate 80, mannitol (E-421), sodium carmellose (E-466), triethyl citrate, and poly(lactic acid) (PLA).
The solvent contains (pre-filled syringe): mannitol, water for injectable preparations, sodium hydroxide (for pH adjustment), and hydrochloric acid (for pH adjustment).
Appearance of the Product and Container Contents
Each container contains a vial with 22.5 mg of leuprorelin acetate, a pre-filled syringe with 2 ml of solvent, an adapter system, and a sterile 20-gauge needle.
Marketing Authorization Holder and Manufacturer
GP-PHARM, S.A.
Pol. Ind. Els Vinyets – Els Fogars Sector 2
Carretera comarcal 244, km22
08777 Sant Quintí de Mediona
Spain
This Medicinal Product is Authorized in the Member States of the European Economic Area under the Following Names:
Spain: Leuprorelina GP-Pharm Depot Trimestral 22.5 mg powder and solvent for prolonged-release injectable suspension
Germany: Lutrate Depot 22.5 mg Pulver und Lösungsmittel zur Herstellung einer Depot-Injektionssuspension
Portugal: Lutrate Depot 22.5 mg / 2 ml powder and vehicle for prolonged-release injectable suspension
Greece: Lutrate Depot 22.5mg Κ?νις και διαλ?της για παρασκευ? ενεσ?μου εναιωρ?ματος παρατεταμ?νης αποδ?σμευσης
Italy: Politrate
Hungary: Politrate Depot 22.5 mg
Austria: Lutrate 3-Monats-Depot 22.5 mg Pulver und Lösungsmittel zur Herstellung einer Depot-injektionssuspension
Czech Republic: Lutrate Depot 22.5mg
Poland: Lutrate Depot
Bulgaria: ?????? ???? 22,5 mg ???? ? ??????????? ?? ??????????? ????????? ? ???????? ?????????????
Date of the Last Revision of this Leaflet:April 2025.
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
This Information is Intended Exclusively for Healthcare Professionals.
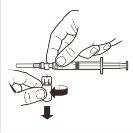
How to Prepare the Injection?
IMPORTANT: Read Carefully Before Administering the Product (Instructions for Use are Also Included in the Tray Containing the Kit Components).
Aseptic technique should be followed during the reconstitution procedure.
Use only the solvent included in the kit.
Once Mixed, the Product Should be Administered Immediately by Single Intramuscular Injection.
This medicinal product is for single use. Any remaining suspension should be discarded.
Check the contents of the kit and ensure that it includes everything mentioned in the leaflet.
The Container Contains:
1 (one) vial of Leuprorelina GP-Pharm Depot 22.5 mg (leuprorelin acetate) powder for injectable suspension
1 (one) pre-filled syringe containing the solvent for the suspension (0.8% mannitol injectable solution)
1 (one) sterile single-use reconstitution device, including 1 (one) sterile needle.
1 |
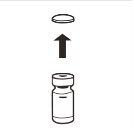
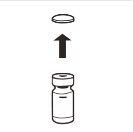
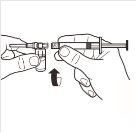
| Completely remove the pressure cap from the top of the vial, so that the rubber stopper is exposed. Confirm that no parts of the pressure cap remain in the vial. |
2 |
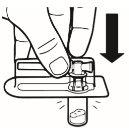
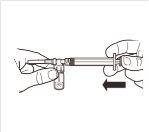
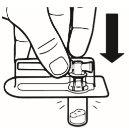
| Place the vial in a vertical position on a table. Remove the blister cover containing the vial adapter (MIXJECT). Do not remove the vial adapter from the blister.Firmly place the blister containing the vial adapter on the top of the vial, perforating the stopperin a fully vertical position. Press gently downwardsuntil it clicks into place. |
3 |
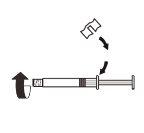
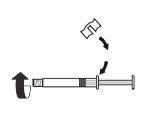
| Fix the white piece to the syringe until it clicks. Unscrewthe rigid cap of the syringe in an anti-clockwise direction. Then, remove the blister from the MIXJECT adapter system. |
4 |
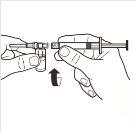
| Connect the syringe to the adapter system by screwing it in a clockwise direction into the lateral opening of the adapter system. To ensure a hermetic connection, screw the syringe gently until it stops. |
5 |
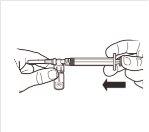
| While keeping the syringe and vial firmly united in a vertical position, slowly push the syringe plunger to transfer all the solvent to the vial. |
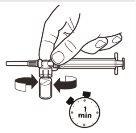
6 |
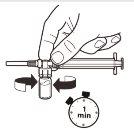
| With the syringe still attached to the vial, gently shake the vial for approximately 1 minuteuntil a uniform milky suspension is obtained.To avoid separation of the suspension, perform the following steps without stopping. |
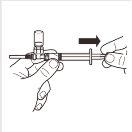
7 |
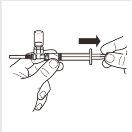
| Turn the MIXJECT adapter system so that the vial is at the top. Firmly hold the MIXJECT adapter system by the syringe and slowly pull the plunger to transfer the contents of the vial to the syringe. Part of the product may accumulate or be deposited on the wall of the vial. This is normal. |
8 |
| Disconnect the syringe from the MIXJECT adapter system. To do this, firmly hold the syringe and turn the vial in a clockwise direction (holding the plastic cap of the adapter system). |
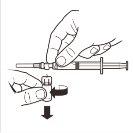
9 |
| Keep the syringe IN A VERTICAL POSITION. With the other hand, remove the needle protector by pulling upwards. Press the plunger slightly to expel the air from the syringe. The syringe containingthe product is ready for immediate administration. |
10 |
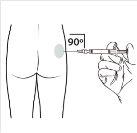
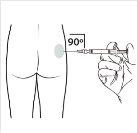
| Administer the intramuscular injection by inserting the needle at a 90-degree angle into the buttock. Ensurethat the entire product is injected.Injection sites should be alternated. |
Instructions for Use
To be Included in the Cover of the Tray Containing the Medicinal Product Kit Components
Leuprorelina GP-Pharm Depot – Instructions for Use
Read Carefully Before Administering the Product
Reconstitute immediately before administration by single intramuscular injection
Use only the solvent included in the commercial kit.
Product intended for a single injection.
Any remaining suspension should be discarded.
1 |
| Completely remove the pressure cap from the top of the vial, so that the rubber stopper is exposed. Confirm that no parts of the pressure cap remain in the vial. |
2 |
| Place the vial in a vertical position on a table. Remove the blister cover containing the vial adapter (MIXJECT). Do not remove the vial adapter from the blister.Firmly place the blister containing the vial adapter on the top of the vial, perforating the stopperin a fully vertical position. Press gently downwardsuntil it clicks into place. |
3 |
| Fix the white piece to the syringe until it clicks. Unscrewthe rigid cap of the syringe in an anti-clockwise direction. Then, remove the blister from the MIXJECT adapter system. |
4 |
| Connect the syringe to the adapter system by screwing it in a clockwise direction into the lateral opening of the adapter system. To ensure a hermetic connection, screw the syringe gently until it stops. |
5 |
| While keeping the syringe and vial firmly united in a vertical position, slowly push the syringe plunger to transfer all the solvent to the vial. |
6 |
| With the syringe still attached to the vial, gently shake the vial for approximately 1 minuteuntil a uniform milky suspension is obtained.To avoid separation of the suspension, perform the following steps without stopping. |
7 |
| Turn the MIXJECT adapter system so that the vial is at the top. Firmly hold the MIXJECT adapter system by the syringe and slowly pull the plunger to transfer the contents of the vial to the syringe. Part of the product may accumulate or be deposited on the wall of the vial. This is normal. |
8 |
| Disconnect the syringe from the MIXJECT adapter system. To do this, firmly hold the syringe and turn the vial in a clockwise direction (holding the plastic cap of the adapter system). |
9 |
| Keep the syringe IN A VERTICAL POSITION. With the other hand, remove the needle protector by pulling upwards. Press the plunger slightly to expel the air from the syringe. The syringe containing the productis ready for immediate administration. |
10 |
| Administer the intramuscular injection by inserting the needle at a 90-degree angle into the buttock. Ensurethat the entire product is injected.Injection sites should be alternated. |
- Country of registration
- Average pharmacy price301.02 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEUPRORELIN GP-PHARM DEPOT TRIMESTRAL 22.5 mg POWDER AND SOLVENT FOR PROLONGED RELEASE INJECTABLE SUSPENSIONDosage form: INJECTABLE, 42 mgActive substance: leuprorelinManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 45 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription requiredDosage form: INJECTABLE, 22.5 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription required
Online doctors for LEUPRORELIN GP-PHARM DEPOT TRIMESTRAL 22.5 mg POWDER AND SOLVENT FOR PROLONGED RELEASE INJECTABLE SUSPENSION
Discuss questions about LEUPRORELIN GP-PHARM DEPOT TRIMESTRAL 22.5 mg POWDER AND SOLVENT FOR PROLONGED RELEASE INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions