
Lutrate Depot

Ask a doctor about a prescription for Lutrate Depot

How to use Lutrate Depot
Leaflet attached to the packaging: patient information
Lutrate Depot, 22.5 mg, powder and solvent for prolonged-release injection suspension
with prolonged release
Leuprorelinum
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Lutrate Depot and what is it used for
- 2. Important information before using Lutrate Depot
- 3. How to use Lutrate Depot
- 4. Possible side effects
- 5. How to store Lutrate Depot
- 6. Contents of the pack and other information
1. What is Lutrate Depot and what is it used for
Lutrate Depot is a medicine that comes as a white powder in a vial, which is converted into an injection suspension. Lutrate Depot contains the active substance - leuprorelin (also known as leuprolide), which belongs to a group of medicines called luteinizing hormone-releasing hormone (LHRH) agonists. These medicines reduce the level of the sex hormone - testosterone. Lutrate Depot is prescribed by a doctor for the palliative treatment of advanced prostate cancer.
2. Important information before using Lutrate Depot
When not to use Lutrate Depot
- if you are allergic to LHRH, LHRH agonist medicines or any of the other ingredients of this medicine (listed in section 6). Allergic reactions may cause: rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue.
- if you have had your testicles removed (orchiectomy)
- in women and children
- if you have spinal cord compression or metastases to the spine, Lutrate Depot should not be used as monotherapy (i.e. as the only medicine) in the treatment of prostate cancer
Warnings and precautions
- Before starting treatment with Lutrate Depot, consult your doctor or pharmacist.
- Your condition may worsen during the first few weeks of treatment, but it should improve as treatment continues. Objective and subjective symptoms include: transient increase in testosterone levels (male sex hormone), hot flashes, bone pain, nervous system disorders (including depression) or difficulty urinating.
- If you think you have had an allergic reaction (shortness of breath, asthma, runny nose, facial swelling, hives, skin rash), stop using the medicine and tell your doctor.
- Tell your doctor if you are at risk or currently have any of the following conditions, as your doctor may need to monitor you more closely:
- if you have unexplained bruising or bleeding or if you generally do not feel well. Although these symptoms are rare, they may indicate a change in the number of red or white blood cells.
- if you have a metabolic disorder
- if you have heart or blood vessel problems
- if you have diabetes
- Tell your doctor if you have had a pituitary tumor (non-cancerous tumor of the pituitary gland) in the past. Cases of pituitary apoplexy (partial destruction of pituitary tissue) have been reported after starting treatment with this type of medicine in patients with pituitary tumors. Symptoms of pituitary apoplexy may include: sudden headache, double vision, blurred or changed vision, and even loss of vision, as well as occasional disturbances of consciousness.
- Tell your doctor if you have bleeding disorders, thrombocytopenia, or if you are taking anticoagulant medicines. It may be necessary to monitor liver function more closely in you, as liver function disorders and jaundice (yellowing of the eyes and skin) have been reported during treatment with leuprorelin.
- During treatment with leuprorelin, spinal fractures, paralysis, decreased blood pressure, and increased blood pressure have been reported.
- In patients receiving Lutrate Depot, depression has been observed, which can be severe. If you experience depressive mood, tell your doctor.
- There have been reports of decreased bone density (brittle or thinning bones) following treatment with leuprorelin. Your doctor may consider using an anti-androgen medicine during treatment with Lutrate Depot. In this case, they must pay special attention to the occurrence of vein inflammation and other symptoms of blood clotting disorders, as well as swelling (of the hands, feet, or joints), as the risk of these increases when an anti-androgen and Lutrate Depot are used together.
- If you have spinal cord compression and/or urinary retention and/or hematuria (blood in the urine), your doctor will start additional treatment if necessary to prevent neurological complications (e.g. tingling in the hands and feet, paralysis) or blockage of the urethra (the tube that carries urine from the bladder out of the body). You should have constant medical care during the first few weeks of treatment.
- Patients may experience metabolic changes (e.g. glucose intolerance or worsening of existing diabetes), weight changes, and cardiovascular disorders.
- During treatment with leuprorelin, patients with metabolic or cardiovascular disorders, especially those with a history of congestive heart failure (a condition in which the heart is unable to supply enough blood to other parts of the body), should be monitored closely.
- Before taking Lutrate Depot, discuss it with your doctor, pharmacist, or nurse if you have fatty liver.
- During treatment, it may be necessary to perform certain blood tests in you to ensure the effectiveness of Lutrate Depot.
- Decreased interest in sex, hot flashes, and sometimes decreased testicle size and function may occur. After stopping treatment with Lutrate Depot, you may regain fertility.
- Since Lutrate Depot may interfere with the results of some laboratory tests, you should tell the doctor ordering the tests that you are taking this medicine.
- If you have a history of seizures, epilepsy, cerebral circulation disorders, central nervous system anomalies or tumors, or if you are taking medicines that may cause seizures, as well as - although to a lesser extent - if you do not belong to any of these groups, seizures may occur during treatment.
- Tell your doctor if you have heart or blood vessel problems, including arrhythmias (irregular heartbeat) or if you are taking medicines for these conditions. Taking Lutrate Depot may worsen arrhythmias.
- If you experience severe or recurring headaches, vision problems, or ringing in the ears, seek medical attention immediately.
- Severe skin rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), have been reported with leuprorelin. If you notice any symptoms of severe skin reactions described in section 4, stop taking leuprorelin and contact your doctor immediately.
Lutrate Depot and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take. It is possible that they can still be used with Lutrate Depot, but the decision will be made by your doctor. Lutrate Depot may interfere with the action of some medicines used to treat arrhythmias (e.g. quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of arrhythmias when used with certain other medicines, e.g. with methadone (used to reduce pain or as one of the medicines used to treat drug addiction), with moxifloxacin (an antibiotic), or with antipsychotic medicines used to treat severe mental illnesses.
Pregnancy and breastfeeding
Lutrate Depot is not intended for use in women. This medicine is contraindicated in pregnant women. Its use during pregnancy may cause spontaneous abortion.
Driving and using machines
The effect of Lutrate Depot on the ability to drive and use machines has not been studied. During treatment, vision disturbances and dizziness may occur. If they occur in you, do not drive or operate machinery.
Lutrate Depot contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per 1 vial, i.e. the medicine is considered "sodium-free".
3. How to use Lutrate Depot
Dose
Lutrate Depot should only be administered by a doctor or nurse who will also prepare the solution.
Adults, including elderly patients
The recommended dose of Lutrate Depot is one injection every three months. After mixing, it is administered as a single intramuscular injection every three months. The injection site should be changed at regular intervals. Lutrate Depot should only be administered intramuscularly. Do not use any other route of administration. The intensity of treatment is determined by the doctor.
Use in children
Lutrate Depot is not indicated for use in children.
Using a higher dose of Lutrate Depot than recommended
It is unlikely that a doctor or nurse will not know the correct dosage. However, if you suspect that you have received a higher dose than you should, tell your doctor immediately so that appropriate action can be taken.
Missing a dose of Lutrate Depot
It is important not to miss a dose of Lutrate Depot. If you forget about an injection, contact your doctor as soon as you remember, and they will administer the next injection.
Stopping treatment with Lutrate Depot
Since treatment with Lutrate Depot is long-term, stopping it may worsen the symptoms of the disease. Therefore, do not stop treatment prematurely without consulting your doctor. If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you experience: sudden wheezing, difficulty breathing, swelling of the eyelids, face or lips, rash or itching of the skin
(especially those covering the whole body). The following side effects have been reported:
Very common(may affect more than 1 in 10 people):
hot flashes and reactions at the injection site
Common(may affect up to 1 in 10 people):
cold sweats, excessive sweating (increased sweating), itching (pruritus), fatigue, insomnia (inability to fall asleep), decreased sex drive, dizziness, flushing, nausea (nausea), diarrhea, decreased appetite, erectile dysfunction, weakness (decreased or lost strength), bone pain, joint pain and reactions at the injection site, such as: pain, hardening, redness (redness of the skin), pain in the urinary system, decreased urine flow, frequent need to urinate, mood changes and depression after long-term use of leuprorelin, changes in liver enzyme activity and increased triglyceride levels in the blood (increased lipid levels in the blood), increased glucose levels in the blood
Uncommon(may affect up to 1 in 100 people):
high cholesterol levels in the blood, sleep disorders, anxiety, taste disorders, tingling (skin sensation disorders), headache, drowsiness (drowsiness), vision disturbances, pleurisy, ringing in the ears (tinnitus), abdominal pain, constipation, lumps, redness, generalized itching (pruritus), night sweats, back pain, muscle pain, neck pain, breast pain, pain in the pelvic area, testicular atrophy, testicular disorders, feeling of heat, mood changes and depression after short-term use of leuprorelin, changes in blood test results and changes in the ECG image (QT interval prolongation) and reactions at the injection site, such as: hives, feeling of heat and bleeding
Frequency not known(frequency cannot be estimated from the available data):
pneumonia, lung disease, idiopathic intracranial hypertension (increased intracranial pressure around the brain, characterized by headache, double vision, and other vision problems, as well as ringing or buzzing in one or both ears), red, flat, plate-like or round spots on the torso, often with blisters in the center, skin peeling, oral, throat, nasal, genital, and eye ulcers - these severe skin rashes may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis), skin redness and itchy rash (toxic skin eruptions), skin reaction causing red spots or patches on the skin, which may look like a target with a dark red center surrounded by lighter red rings (erythema multiforme)
Reporting side effects
If you experience any side effects, including any possible side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative in Poland.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Lutrate Depot
Doctors and pharmacists are informed about how to store this medicine.
Store in a place out of sight and reach of children.
Do not store above 25°C. Do not freeze.
Do not use this medicine after the expiry date stated on the carton, vial, and ampoule-needle after "EXP". The expiry date on the ampoule-needle is the same as on the vial. The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Lutrate Depot contains
The active substance of the medicine is leuprorelin acetate. Each vial contains 22.5 mg of leuprorelin acetate. The reconstituted medicine has a concentration of 11.25 mg/ml. The other ingredients are:
powder (vial): poly(lactic acid) (PLA), triethyl citrate, mannitol, sodium carmellose, polysorbate 80
solvent (in ampoule-needle): mannitol, hydrochloric acid (to adjust pH), sodium hydroxide (to adjust pH), water for injections
What Lutrate Depot looks like and contents of the pack
Each pack contains a vial containing 22.5 mg of leuprorelin acetate, 1 ampoule-needle containing 2 ml of solvent, 1 vial connector, and 1 sterile needle for injection.
Marketing authorization holder
+pharma arzneimittel gmbh
Hafnerstrasse 211
8054 Graz
Austria
Manufacturer
GP-Pharm S.A.
Poligono Industrial Els Vinyets - Els Fogars, Sector 2
Carretera Comarcal C -244, Km. 22
08777 Sant Quintí de Mediona (Barcelona)
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria
Lutrate Depot 22.5 mg Powder and solvent for prolonged-release injection suspension
Bulgaria
Лутрат Депо 22,5 mg прах и разтворител за инжекционна суспензия с удължено освобождаване
Czech Republic
Lutrate Depot 22.5 mg
Greece
Lutrate Depot 22.5 mg Κόνις και διαλύτης για παρασκευή ενεσίμου εναιωρήματος παρατεταμένης αποδέσμευσης
Spain
Leuprorelina GP-Pharm Depot Trimestral 22.5 mg polvo y disolvente para suspensión de liberación prolongada inyectable
Germany
Lutrate Depot 22.5 mg Pulver und Lösungsmittel zur Herstellung einer Depot-Injektionssuspension
Poland
Lutrate Depot
Portugal
Lutrate Depot 22.5 mg / 2 ml pó e veículo para suspensão injectável de libertação prolongada
Hungary
Politrate Depot 22.5 mg
Italy
Politrate
To obtain more detailed information on this medicine, contact the representative of the marketing authorization holder in Poland:
+pharma Polska sp. z o.o.
ul. Podgórska 34
31-536 Kraków, Poland
tel.: +48 12 262 32 36
e-mail: [email protected]
Date of last revision of the leaflet:May 2025
Information intended for healthcare professionals only
Preparation of the medicine for injection
IMPORTANT: Read carefully before administering the product (“Instructions for use” are also
included on the tray containing the components of the product kit). During the preparation procedure, follow the principles of asepsis.
Use only the diluent provided with the kit.
After mixing, administer it immediately as a single intramuscular injection. The product is intended for single use only. Any remaining suspension should be disposed of.
Check the contents of the kit and make sure it contains all the parts listed in the leaflet.
The kit contains:
- 1 (one) vial of Lutrate Depot containing 22.5 mg of leuprorelin acetate in the form of a powder for injection suspension
- 1 (one) ampoule-needle containing solvent for injection suspension (0.8% mannitol injection solution)
- 1 (one) vial connector needed to reconstitute the medicine, along with 1 (one) sterile needle for injection.
1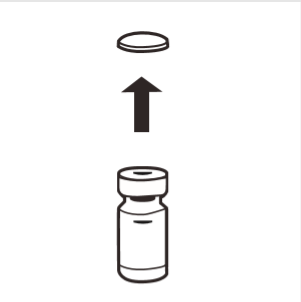 | Completely remove the removable cap from the top of the vial, exposing the rubber stopper. Make sure that no parts of the removable cap remain on the vial. |
2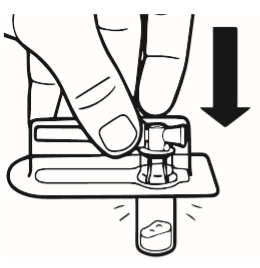 | Place the vial on a table, in a vertical position. Tear off the blister pack containing the vial connector (MIXJECT). Do not remove the vial connector from the blister pack. Place the blister pack with the vial connector firmly on the top of the vial, piercing the vial while it is in a completely vertical position. Gently press until you feel the connector click into place. |
3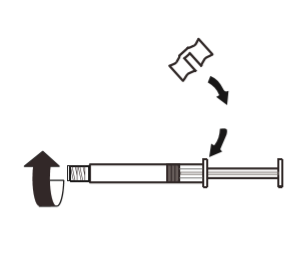 | Attach the white handle to the syringe so that it clicks into place. Unscrew the rubber cap of the syringe in the opposite direction of the arrow. Then remove the blister pack from the MIXJECT system. |
4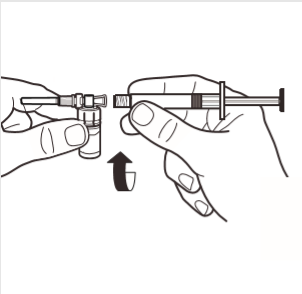 | Connect the syringe to the ampoule adapter by screwing it in in the direction of the arrow on the side of the adapter. Gently screw the syringe until it stops turning to ensure a secure connection. |
5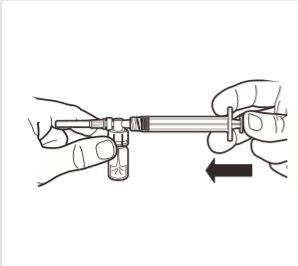 | While holding the syringe and ampoule in a vertical position, slowly press the plunger to transfer all of the diluent into the ampoule. |
6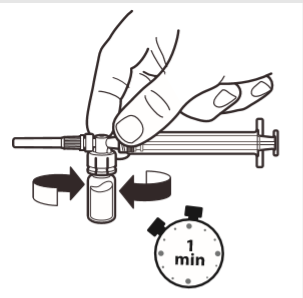 | With the syringe still connected to the ampoule, gently shake the ampoule for about one minute until a uniform milky white suspension is obtained. To avoid settling of the suspension, proceed to the next steps immediately. |
7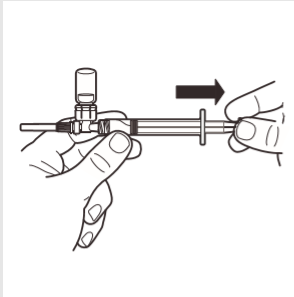 | Invert the MIXJECT system so that the ampoule is on top. Firmly grasp the MIXJECT system by the syringe and slowly pull back the plunger to fill the syringe with the prepared product. Some product may settle or stick to the ampoule walls. This is a normal phenomenon. |
8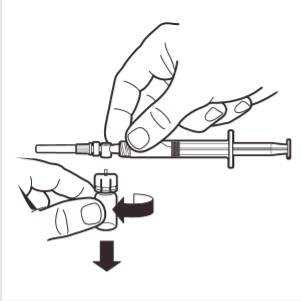 | Disconnect the ampoule adapter from the MIXJECT system connected to the syringe: firmly grasp the syringe and turn the ampoule (holding the plastic adapter cap) in the direction of the arrow. |
9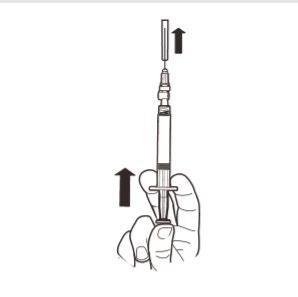 | Hold the syringe VERTICALLY. With your other hand, pull the needle cap upwards. Press the plunger to remove any air from the syringe. The syringe containing the product is now ready for immediate administration. |
10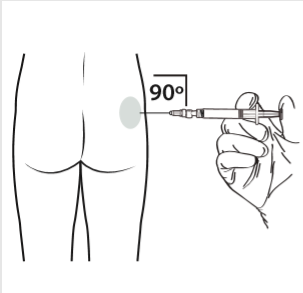 | Administer the intramuscular injection by inserting the needle at a 90-degree angle in the buttocks area. Ensure that the entire amount of product is injected. The injection sites should be changed. |
Instructions for use
Lutrate Depot - instructions for use
Read carefully before administering the product.
Prepare for use immediately before administration in the form of a single intramuscular injection.
Use only the diluent provided with the kit.
The product is intended for single use only. Any remaining suspension should be disposed of.
1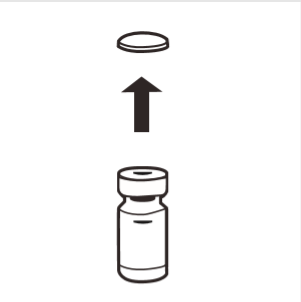 | Completely remove the removable cap from the top of the vial, exposing the rubber stopper. Make sure that no parts of the removable cap remain on the vial. |
2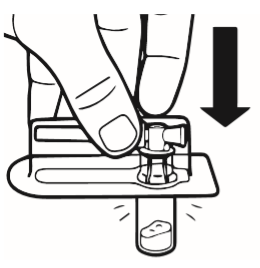 | Place the vial on a table, in a vertical position. Tear off the blister pack containing the vial connector (MIXJECT). Do not remove the vial connector from the blister pack. Place the blister pack with the vial connector firmly on the top of the vial, piercing the vial while it is in a completely vertical position. Gently press until you feel the connector click into place. |
3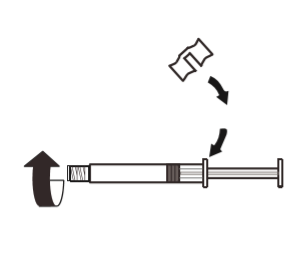 | Attach the white handle to the syringe so that it clicks into place. Unscrew the rubber cap of the syringe in the opposite direction of the arrow. Then remove the blister pack from the MIXJECT system. |
4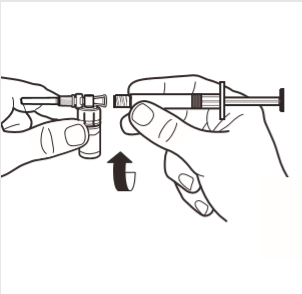 | Connect the syringe to the ampoule adapter by screwing it in in the direction of the arrow on the side of the adapter. Gently screw the syringe until it stops turning to ensure a secure connection. |
5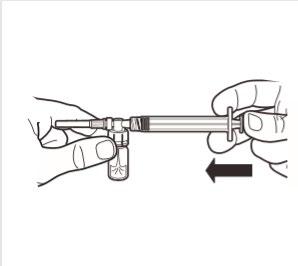 | While holding the syringe and ampoule in a vertical position, slowly press the plunger to transfer all of the diluent into the ampoule. |
6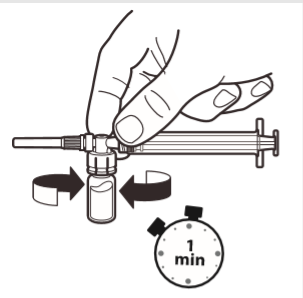 | With the syringe still connected to the ampoule, gently shake the ampoule for about one minute until a uniform milky white suspension is obtained. To avoid settling of the suspension, proceed to the next steps immediately. |
7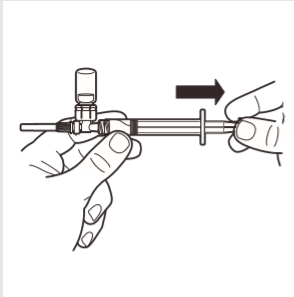 | Invert the MIXJECT system so that the ampoule is on top. Firmly grasp the MIXJECT system by the syringe and slowly pull back the plunger to fill the syringe with the prepared product. Some product may settle or stick to the ampoule walls. This is a normal phenomenon. |
8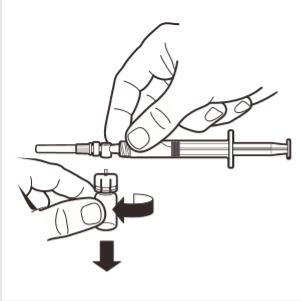 | Disconnect the ampoule adapter from the MIXJECT system connected to the syringe: firmly grasp the syringe and turn the ampoule (holding the plastic adapter cap) in the direction of the arrow. |
9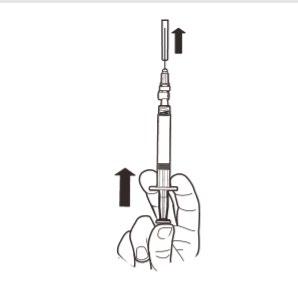 | Hold the syringe VERTICALLY. With your other hand, pull the needle cap upwards. Press the plunger to remove any air from the syringe. The syringe containing the product is now ready for immediate administration. |
10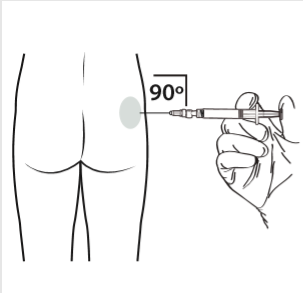 | Administer the intramuscular injection by inserting the needle at a 90-degree angle in the buttocks area. Ensure that the entire amount of product is injected. The injection sites should be changed. |
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGP-PHARM S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Lutrate DepotDosage form: Powder, 22.5 mgActive substance: leuprorelinPrescription requiredDosage form: Powder, 45 mgActive substance: leuprorelinPrescription requiredDosage form: Powder, 7.5 mgActive substance: leuprorelinPrescription required
Alternatives to Lutrate Depot in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Lutrate Depot in Spain
Alternative to Lutrate Depot in Ukraine
Online doctors for Lutrate Depot
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Lutrate Depot – subject to medical assessment and local rules.











