

Leuprostin

Ask a doctor about a prescription for Leuprostin

How to use Leuprostin
Package Leaflet: Information for the Patient
Leuprostin, 3.6 mg, Implant
Leuprorelin
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
Keep this package leaflet, you may need to read it again.
In case of any doubts, consult a doctor, pharmacist, or nurse.
This medicine has been prescribed specifically for you. Do not pass it on to others.
The medicine may harm another person, even if their symptoms are the same.
If the patient experiences any side effects, including those not listed in this leaflet, tell the doctor, pharmacist, or nurse.
See section 4.
Table of Contents of the Package Leaflet:
- 1. What is Leuprostin and what is it used for
- 2. Important information before using Leuprostin
- 3. How to use Leuprostin
- 4. Possible side effects
- 5. How to store Leuprostin
- 6. Contents of the pack and other information
1. What is Leuprostin and what is it used for
The active substance of Leuprostin (leuprorelin acetate) belongs to a group of inhibitors of certain sex hormones.
Leuprostin acts on the pituitary gland, initially stimulating and then inhibiting the production of hormones that regulate the production of male sex hormones in the testes.
This means that the concentration of sex hormones decreases and remains at the same level during treatment. After stopping the use of Leuprostin, the hormone levels return to normal.
Leuprostin is used in the symptomatic treatment of advanced prostate cancer that is dependent on hormone activity.
Leuprostin is also used in the treatment of locally advanced prostate cancer and prostate cancer limited to the prostate, dependent on hormone activity, during or after radiotherapy.
2. Important information before using Leuprostin
When not to use Leuprostin
if the patient is allergic to leuprorelin or any of the other ingredients of this medicine (listed in section 6);
if the patient is allergic to substances similar to leuprorelin, such as goserelin or buserelin;
if the cancer diagnosed in the patient is not dependent on hormone activity;
in women and children.
Warnings and precautions
Before starting treatment with Leuprostin, discuss it with a doctor or nurse:
if the patient has high blood pressure. In this case, the doctor will closely monitor the patient's condition.
if the patient has undergone removal of both testes. In this case, Leuprostin will not cause further reduction in the level of male sex hormone in the blood.
if the patient still has neurological symptoms (spinal cord compression, metastases to the spine) or experiences discomfort while urinating due to changes in the urinary tract before starting treatment. Tell the doctor immediately, who will closely monitor the patient's condition in the first weeks of treatment in a hospital setting, if possible.
if the symptoms of the disease recur (i.e., pain, difficulty urinating, or weakness in the legs during long-term use of Leuprostin). In this case, the doctor will regularly check the effectiveness of the treatment by performing appropriate tests (digital rectal examination, imaging tests) and monitoring blood parameters (phosphatase activity and prostate-specific antigen (PSA) levels, as well as testosterone levels).
if the patient is at risk of developing osteoporosis. If possible, the doctor may recommend additional medication to prevent bone loss.
if the patient has diabetes. In this case, the doctor will closely monitor the patient's condition.
if the patient has fatty liver disease (a condition in which excess fat accumulates in the liver)
If the patient experiences severe or recurring headaches, vision problems, or ringing in the ears, seek medical attention immediately.
Depression has been reported in patients using Leuprostin. If the patient experiences depressive mood during treatment with Leuprostin, tell the doctor.
Tell the doctor about any heart or blood vessel disorders, including arrhythmias or medications taken for this purpose. During treatment with Leuprostin, the risk of arrhythmias may increase.
Severe skin reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), have been reported with the use of leuprorelin. If any symptoms of severe skin reactions occur, discontinue leuprorelin and seek medical attention immediately.
Leuprostin and other medicines
Tell the doctor, pharmacist, or nurse about all medicines the patient is currently taking or has recently taken, as well as any medicines the patient plans to take.
Leuprostin may interact with certain medicines used to treat arrhythmias (such as quinidine, procainamide, amiodarone, and sotalol) or increase the risk of arrhythmias when used with certain other medicines [e.g., methadone (used as a pain reliever and in detoxification of drug addicts), moxifloxacin (an antibiotic), antipsychotic medicines (used to treat severe mental disorders)].
Children and adolescents
Leuprostin is intended for adult patients only.
Pregnancy and breastfeeding
Leuprostin is intended for men only.
Driving and using machines
Both the medicine and the underlying cancer can cause fatigue. This is more likely if the patient consumes alcoholic beverages. If this applies to the patient, they should not drive or operate machinerywithout consulting a doctor .
3. How to use Leuprostin
Administration of Leuprostin
Clean the injection site.
A local anesthetic may be used to alleviate pain during injection of the implant.
Leuprostin is administered subcutaneously in the abdominal wall.
Leuprostin should only be administered by a doctor or nurse who will also prepare the product.
How much to use
The recommended doseis 1 implantcontaining 3.6 mg of leuprorelin, administered monthly.
Follow the doctor's instructions regarding the timing of administration and intervals between injections of the implant.
Leuprostin is administered monthly. After the second administration, the next injection of the implant can be delayed by up to 2 weeks in exceptional cases. The effectiveness of the treatment usually remains unchanged in most patients.
An implant contained in one prefilled syringe is injected.
The prefilled syringe contains one implant containing 3.6 mg of leuprorelin.
Blood tests
The doctor will order regular blood tests to check the effectiveness of the medicine.
After 3 months of therapy, the doctor usually evaluates whether Leuprostin is effective in treating prostate cancer in the patient. To do this, it is necessary to check the level of prostate-specific antigen (PSA) and testosterone in the blood.
Duration of treatment
The duration of treatment is determined by the doctor. Treatment should be continued even if the symptoms of the disease have disappeared or the disease has progressed.
Prostate cancer can be treated with Leuprostin for several years. Therefore, it can be used continuously if it is effective and well-tolerated by the patient. The doctor will order tests at regular intervals to evaluate the effectiveness of the treatment, especially in case of recurrence:
- pain
- difficulty urinating
- weakness in the legs.
More frequent administration of Leuprostin than recommended
It is unlikely that the doctor or nurse will administer an overdose of the medicine.
In case of accidental administration of a larger amount of medicine, the doctor will monitor the patient and, if necessary, provide appropriate treatment.
Missing a dose of Leuprostin
If the patient suspects that they have not received their dose of the medicine after a month, they should talk to their doctor.
Stopping treatment with Leuprostin
If the treatment is stopped without the doctor's recommendation, the symptoms associated with the disease may worsen.
Therefore, treatment should not be stopped prematurely without consulting a doctor.
In case of any further doubts about the use of this medicine, consult a doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Leuprostin can cause side effects, although not everybody gets them.
If the patient experiences any of the following serious side effects, seek medical attention immediately:
Allergic reactions(anaphylactic reactions) with sudden onset of symptoms such as:
- hot flashes, rash, itching, or hives on the skin and/or mucous membranes
- swelling of the face, lips, tongue, or other parts of the body
- shortness of breath, wheezing, or difficulty breathing
- decreased blood pressure, rapid heartbeat, seizures, and, in severe cases, life-threatening circulatory failure. Swelling and pain in some parts of the bodydue to the formation of a blood clot in a vein. Difficulty breathing, chest pain, fainting, rapid heartbeat, bluish discoloration of the skin, and changes in skin colordue to a blood clot in the lungs.
These are rare side effects (may occur in less than 1 in 1000 people).
A common phenomenon is the initial short-term increase in the level of male sex hormone (testosterone) in the blood. This may cause a temporary worsening of symptoms associated with the disease, such as:
bone pain
difficulty urinating due to narrowing of the urinary tract
spinal cord compression
weakness in the legs
swelling of tissues due to fluid retention (lymphedema)
The worsening of these symptoms usually resolves on its own without the need to stop using Leuprostin.
At the beginning of treatment, the doctor may recommend taking an appropriate hormone antagonist (so-called antiandrogen) to alleviate possible disorders associated with the initial increase in testosterone levels.
During treatment, the level of testosterone decreases to very low values. As a result, some patients may experience the following side effects:
Very common(may occur in more than 1 in 10 people):
bone pain
hot flashes with sudden sweating
decreased sex drive and potency
excessive sweating
Common(may occur in less than 1 in 10 people):
depression, mood changes
abnormal sensations, such as tingling or numbness
increased need to urinate at night
increased need to urinate during the day
difficult and painful urination
increased appetite
sleep disorders
Uncommon(may occur in less than 1 in 100 people):
decreased appetite
decreased or increased blood sugar levels
headache
dizziness
changes in blood pressure (decreased or increased blood pressure)
breathing difficulties
diarrhea
hair loss
dry skin and mucous membranes
night sweats
inability to fully empty the bladder
decreased testicle size
testicle pain
breast enlargement in men
weight gain
weight loss
increased activity of liver enzymes (ALT, AST, gamma-GT) and other enzymes (LDH, alkaline phosphatase)
Rare(may occur in less than 1 in 10,000 people):
generalized allergic reactions (fever, skin rash, itching, increased white blood cell count [eosinophilia])
transient changes in taste
nausea and/or vomiting
joint and/or back pain, muscle disorders
swelling
feeling of fatigue
local skin reactions, such as redness or hardening, pain, swelling, and itching at the injection site, which usually resolves even with continued treatment; in individual cases, an ulcer
pituitary apoplexy after the first administration of the medicine in patients with pituitary adenoma (as with other medicines in this group)
Frequency not known(frequency cannot be estimated from the available data):
non-inflammatory lung disease (interstitial lung disease), mainly reported in Japan
pneumonia, lung disease
individual cases of ulceration at the injection site
changes in ECG (QT interval prolongation)
seizures
idiopathic intracranial hypertension (increased intracranial pressure around the brain, characterized by headache, double vision, and other vision problems, as well as ringing in the ears)
red, flat, round, or oval patches on the torso, often with blisters in the center, peeling skin, ulcers in the mouth, throat, nose, genitals, and eyes.
These severe skin reactions may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis).
redness of the skin and itchy rash. (toxic skin eruptions)
skin reaction causing the appearance of red dots or patches on the skin, which may look like a target with a dark red center surrounded by lighter red rings (erythema multiforme).
Special information
The effectiveness of Leuprostin can be monitored by measuring the levels of testosterone and prostate-specific antigen (PSA) in the blood, as well as phosphatase activity. The level of testosterone increases at the beginning of treatment and decreases within the next 2 weeks. After 2 to 4 weeks, the level of testosterone reaches the value observed in patients after removal of both testes and remains at this level throughout the treatment period.
At the beginning of treatment, it is possible to have a transient increase in phosphatase activity in the blood.
After a few weeks, this activity returns to normal or near-normal values.
The decrease in testosterone levels that occurs after removal of the testes or as a result of taking medicines that suppress sex hormones (such as Leuprostin) may lead to a decrease in bone density with a higher risk of fractures (see "Warnings and precautions").
The decrease in bone density is more noticeable after removal of the testes than after administration of Leuprostin. The doctor may recommend taking additional medication that regulates calcium metabolism (from the group of so-called bisphosphonates).
Reporting side effects
If side effects occur, including those not listed in this leaflet, tell the doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw
tel.: + 48 22 49 21 301, fax: + 48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Leuprostin
Store this medicine out of sight and reach of children.
Do not use the medicine after the expiry date stated on the carton, blister, and syringe label after EXP. The expiry date refers to the last day of the month.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Ask the pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Leuprostin contains
- -The active substance is leuprorelin (as leuprorelin acetate). Each implant contains 3.6 mg of leuprorelin (as leuprorelin acetate).
- The other ingredient is: copolymer of lactic acid and glycolic acid (1:1)
What Leuprostin looks like and contents of the pack
Prefilled syringe made of polycarbonate (PC) with a plunger made of Acrylonitrile-Butadiene-Styrene (ABS) copolymer and a needle, placed in a bag made of PET/Aluminum/PE film with a desiccant, in a cardboard box.
Packs contain:
1 prefilled syringe with 1 implant
2 prefilled syringes, each with 1 implant
3 prefilled syringes, each with 1 implant
Marketing authorization holder and manufacturer
Marketing authorization holder
Sandoz GmbH
Biochemiestrasse 10
6250 Kundl, Austria
Manufacturer
EVER Pharma Jena GmbH
Otto-Schott-Strasse 15
07745 Jena, Germany
Sandoz GmbH
Biochemiestrasse 10
6250 Kundl, Austria
EVER Pharma Jena GmbH
Brüsseler Strasse 18
07747 Jena, Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria:
Leuprorelin Sandoz 3.6 mg - Implantat für 1 Monat
Denmark:
Leuprorelin Sandoz
Germany:
Leuprorelin HEXAL 3.6 mg
Hungary:
Leuprorelin Sandoz 3.6mg implantátum
Italy:
LEPTOPROL
Norway:
Leuprorelin Sandoz 3.6 mg implantat
Poland:
LEUPROSTIN
Sweden:
Leuprorelin Sandoz 3.6 mg implantat
Slovakia:
Leuprorelin Sandoz 3.6 mg implantát
For more information about this medicine, contact:
Sandoz Polska Sp. z o.o.
ul. Domaniewska 50 C
02-672 Warsaw
tel. 22 209 70 00
Date of last revision of the leaflet:12/2024
Sandoz logo
-----------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only
Read the instructions carefully, as the applicator of this medicinal product may differ from others used previously.
Instructions for use
- 1. Clean the injection site located on the abdominal wall below the navel.
- 2. Remove the applicator from the sterile packaging and check if the implant is visible inside (see figure in the frame). For certainty, the applicator should be examined under light or gently shaken.
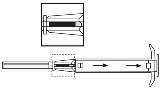
- 3. Pull the applicator plunger completely back, until the second notch appears.
Warning:The plunger can only be moved forward to inject the implant if it has been completely pulled back!
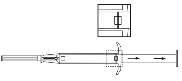
- 4. Remove the needle shield.
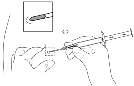
- 5. Hold the applicator body with one hand. With the other hand, pinch the skin of the abdominal wall below the navel of the patient. See figure. Holding the obliquely cut needle tip upwards, insert the entire needleat a slight angle into the subcutaneous tissue, almost parallel to the skin surface.
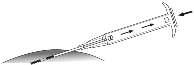
- 6. Carefully withdrawthe applicator about 1 cm back, creating a puncture canal for the implant.
- 7. Inject the implant into the puncture canal by completelypressing the plunger until it clicks into place with an audible click.
- 8. Remove the needle. To ensure that the implant has been injected correctly, check if the white end of the plunger is visible in the needle tip.
Information on dosing can be found in section 3 "How to use Leuprostin".
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterEVER Pharma Jena GmbH EVER Pharma Jena GmbH Sandoz GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LeuprostinDosage form: Powder, 22.5 mgActive substance: leuprorelinPrescription requiredDosage form: Powder, 45 mgActive substance: leuprorelinPrescription requiredDosage form: Powder, 7.5 mgActive substance: leuprorelinPrescription required
Alternatives to Leuprostin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Leuprostin in Spain
Alternative to Leuprostin in Ukraine
Online doctors for Leuprostin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Leuprostin – subject to medical assessment and local rules.











