CAMCEVI 42 mg PROLONGED-RELEASE INJECTABLE SUSPENSION

How to use CAMCEVI 42 mg PROLONGED-RELEASE INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
CAMCEVI 42 mg prolonged-release injectable suspension
leuprorelin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is CAMCEVI and what is it used for
- What you need to know before you are given CAMCEVI
- How CAMCEVI will be given to you
- Possible side effects
- Storing CAMCEVI
- Contents of the pack and other information
1. What is CAMCEVI and what is it used for
The active substance of CAMCEVI is leuprorelin, a GnRH agonist (a synthetic version of a natural hormone known as gonadotropin-releasing hormone), and it works in the same way as the natural hormone to reduce the levels of the male sex hormone testosterone in the body.
Prostate cancer is sensitive to hormones, such as testosterone. Reducing testosterone levels helps control the development of the cancer.
CAMCEVI is used to treat adult menwith:
- metastatic hormone-dependent prostate cancer, and
- high-risk non-metastatic hormone-dependent prostate cancerin combination with radiotherapy.
2. What you need to know before you are given CAMCEVI
DO NOT use CAMCEVI:
- if you are a woman or a child under 18 years of age;
- if you are allergicto leuprorelin or to similar medicines that affect sex hormones(GnRH agonists). Your doctor will help you identify them if necessary.
- if you are allergic to any of the other ingredientsof this medicine (listed in section 6);
- after surgical removal of your testicles. This medicine cannot help reduce your testosterone levels when you do not have testicles;
- as the only treatment if you have symptoms related to pressure on the spinal cord or a tumor in the spine. In this case, CAMCEVI can only be used in combination with other medicines for prostate cancer.
Warnings and precautions
Seek medical help immediatelyif you start to suffer from:
- sudden headache;
- vomiting;
- loss of vision or double vision;
- loss of ability to move the eye muscles in the eye area;
- altered mental state;
- early symptoms of heart failure, including:
- fatigue
- swelling in the ankles
- increased need to urinate at night
- more severe symptoms, such as rapid breathing, chest pain, and fainting.
These may be signs of a condition called pituitary apoplexy, which involves bleeding in the pituitary gland or poor blood circulation in the pituitary gland at the base of the brain. Pituitary apoplexy can occur due to a tumor in the pituitary gland and can rarely occur after starting treatment. Most cases occur within 2 weeks of the first dose, and in some cases, within the first hour.
Talk to your doctor, pharmacist, or nurse before starting CAMCEVI if
- you develop signs and symptoms of cardiovascular problems such as rapid and irregular heartbeats. These rapid heartbeats can cause you to faint or have seizures (convulsions);
- you have any heart or vascular problems, including heart rhythm problems (arrhythmias), or if you take medicines to correct these conditions. The risk of heart rhythm problems may worsen with the use of CAMCEVI. Your doctor may monitor your heart with an electrocardiogram (ECG);
- you have prostate cancer that has spread to the spine or brain. Your doctor will monitor you more closely during the first weeks of treatment.
- if you suffer from diabetes mellitus(high blood sugar levels). CAMCEVI may worsen existing diabetes, and therefore, diabetic patients should undergo frequent blood sugar level tests.
Talk to your doctor, pharmacist, or nurse during treatment with CAMCEVI if
- you have a heart attack. Symptoms include chest pain, difficulty breathing, dizziness, and sweating;
- you have a stroke. Symptoms include facial muscle drooping, inability to raise your arms, and slurred speech;
- you have a bone fracture. Treatment with CAMCEVI may increase the risk of fractures due to osteoporosis (reduced bone density);
- you have a seizure (convulsions);
- you notice an increase in your blood sugar levels. Your doctor will monitor your blood sugar levels during treatment;
- you have difficulty urinating. You may have blocked urinary tract. Your doctor will monitor you closely during the first weeks of treatment;
- you develop symptoms of spinal compressionsuch as pain, numbness, or weakness in arms, hands, legs, or feet. Your doctor will monitor you closely during the first weeks of treatment.
Problems you may experience during the first few weeks of treatment
Usually, during the first few weeks of treatment, there is a brief increase in the male sex hormone testosterone in the blood. This can cause a temporary worsening of symptoms related to the disease and also the appearance of new symptoms never experienced before. These symptoms may include:
- bone pain;
- urination problems, pain, numbness, or weakness in arms, hands, legs, or feet, or loss of control of the bladder or sphincters, which can be symptoms of spinal compression;
- blood in the urine.
These symptoms usually decrease with continued treatment. If not, contact your doctor.
You may be treated with another medicine before starting CAMCEVI to help reduce the initial increase in blood testosterone levels. You may also need to continue with this other medicine for a few weeks after starting CAMCEVI therapy.
If you do not improve with CAMCEVI
Some patients have tumors that are not sensitive to lower testosterone levels. Talk to your doctor if you think the effect of CAMCEVI is not as expected.
Other medicines and CAMCEVI
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
CAMCEVI may interfere with some medicines used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, sotalol, dofetilide, and ibutilide) or may increase the risk of heart rhythm problems when used with certain medicines, such as methadone (used to relieve pain and as a substitute for heroin in drug addiction treatment), moxifloxacin (an antibiotic), and antipsychotics used for severe mental illnesses.
Pregnancy and breastfeeding
This medicine is not for women.
Driving and using machines
During treatment with CAMCEVI, you may experience fatigue, dizziness, and visual disturbances. If you experience any of these side effects, do not drive, use tools, or operate machines.
3. How CAMCEVI will be given to you
CAMCEVI is given as a single injection under the skin (subcutaneously) every six months, administered by your doctor or nurse.
This medicine should only be administered by your doctoror a nurse, who will ensure that it is injected under the skin and not into a vein.
After the injection, the medicine solidifies and then gradually releases leuprorelin into the body over a period of 6 months.
In combination with radiotherapy
For localized high-risk or locally advanced prostate cancer, this medicine should be used before or at the same time as radiotherapy. Localized high-riskmeans that the cancer is likely to spread outside the prostate to nearby tissues, becoming locally advanced. Locally advancedmeans that the cancer has spread outside the pelvis to nearby tissues such as lymph nodes.
Monitoring your treatment
Your doctor will monitor your response to treatment through blood tests, including prostate-specific antigen (PSA).
If you are given too much CAMCEVI
Since the injection is given by your doctor or trained personnel, an overdose is unlikely. If you are accidentally given too much medicine, your doctor will monitor you and implement any necessary additional treatment.
If you miss a dose of CAMCEVI
Talk to your doctor if you think you have missed your six-monthly administration of CAMCEVI.
Effects of stopping treatment with CAMCEVI
As a rule, treatment of prostate cancer with CAMCEVI is long-term. Therefore, treatment should not be stopped too early, even if you notice relief from symptoms or if they disappear completely. If treatment is stopped prematurely, your symptoms may recur. You should not stop treatment prematurely without consulting your doctor first.
If you have any questions related to the use of this medicine, talk to your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek medical help immediatelyif you start to suffer from:
- sudden headache;
- vomiting;
- loss of vision or double vision;
- loss of ability to move the eye muscles in the eye area;
- altered mental state;
- early symptoms of heart failure, including:
- fatigue
- swelling in the ankles
- increased need to urinate at night
- more severe symptoms, such as rapid breathing, chest pain, and fainting.
These may be signs of a condition called pituitary apoplexy, which involves bleeding in the pituitary gland or poor blood circulation in the pituitary gland at the base of the brain. Pituitary apoplexy can occur due to a tumor in the pituitary gland and can rarely occur after starting treatment. Most cases occur within 2 weeks of the first dose, and in some cases, within the first hour.
Initial side effects
Usually, during the first week of treatment, there is a brief increase in the male sex hormone testosterone in the blood. This can cause a temporary worsening of symptoms related to the disease and also the appearance of new symptoms never experienced before. These symptoms may include:
- bone pain;
- urination problems, pain, numbness, or weakness in arms, hands, legs, or feet, or loss of control of the bladder or sphincters, which can be symptoms of spinal compression;
- blood in the urine.
Your doctor may prescribe another medicine when starting CAMCEVI therapy to reduce some of the initial side effects (see also section 2, Problems you may experience during the first few weeks of treatment).
Side effects at the injection site
After the injection, you may experience the following adverse reactions around the injection site:
- burning and numbness immediately after the injection (very common: may affect more than 1 in 10 people)
- pain, bruising, and prickling sensation after the injection (common: may affect up to 1 in 10 people)
- itching and hardening of the skin around the injection site (uncommon: may affect up to 1 in 100 people)
- skin damage or pain at the injection site (rare: may affect up to 1 in 1,000 people)
- dead tissue at the injection site (very rare, may affect up to 1 in 10,000 people).
These reactions are mild and do not last long. They only occur at the time of injection. If you experience any of these side effects, talk to your doctor.
Very common side effects(may affect more than 1 in 10 people)
- hot flushes
- bruising and/or redness of the skin
- fatigue.
Common side effects(may affect up to 1 in 10 people)
- common cold symptoms (nasopharyngitis)
- nausea, diarrhea, inflammation of the stomach and intestines (gastroenteritis/colitis)
- itching
- night sweats
- joint pain, pain in arms and legs, discomfort, and muscle pain
- increased need to urinate, difficulty urinating, pain while urinating, not urinating enough, or decreased need to urinate
- breast tenderness and/or swelling, decreased testicle size, testicle pain, infertility, erectile dysfunction, reduced penis size
- exaggerated trembling episodes with high fever (rigidity), weakness, general malaise
- changes in blood test results (prolonged bleeding time, changes in blood values, decreased red blood cell count).
Uncommon side effects(may affect up to 1 in 100 people)
- urinary tract infection, local skin infection
- worsening of diabetes mellitus
- abnormal dreams, depression, decreased libido (sex drive)
- dizziness, headache, partial or total loss of sensitivity in a part of the body, insomnia, abnormal changes in taste and/or smell
- dizziness, loss of balance, and vertigo
- changes in electrocardiogram results
- prolongation of the QT interval
- heart attack. Symptoms include chest pain, difficulty breathing, swelling, and sweating
- high or low blood pressure
- runny nose, shortness of breath
- constipation, dry mouth, indigestion, symptoms of having a full stomach, stomach pain, belching, nausea, vomiting, sensation of burning in the stomach (dyspepsia), vomiting
- feeling of humidity and sweating
- back pain, muscle cramps
- bladder spasms, blood in the urine, overactive bladder (need to urinate before the bladder is full), inability to urinate
- breast enlargement, impotence, testicular problems (e.g., swelling, redness, or high temperature in the scrotum, pain, and discomfort in the pelvic area)
- sleepiness (lethargy), pain, fever
- changes in blood test results, weight gain.
Rare side effects(may affect up to 1 in 1,000 people)
- abnormal involuntary movements;
- fainting, collapse
- flatulence, belching
- hair loss, skin pimples
- breast pain.
Frequency not known(frequency cannot be estimated from the available data)
- inflammation of the lungs (interstitial lung disease)
- idiopathic intracranial hypertension (increased pressure around the brain characterized by headaches, double vision, and other visual symptoms, ringing in one or both ears).
The following are serious allergic reactions reported in relation to medicines that belong to the same group as CAMCEVI
- difficulty breathing or dizziness (rare).
The following side effects were reported in relation to other medicines that contain leuprorelin
- swelling of hands and feet (edema)
- symptoms of pulmonary embolism (a blood clot in the blood vessels of the lungs), including chest pain, difficulty breathing, and coughing up blood
- notably rapid, strong, or irregular heartbeat;
- muscle weakness
- chills
- skin rash
- poor memory
- visual impairment
- loss of muscle mass/muscle tissue loss after prolonged use
- a bone condition called osteoporosis in which bones become fragile and brittle, with a higher risk of bone fractures.
The following side effect has been reported in relation to medicines that belong to the same group as CAMCEVI
- seizures.
Reporting of side effects
If you experience side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing CAMCEVI
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C to 8°C).
Keep the container in the outer carton to protect it from light.
Before using, let CAMCEVI reach room temperature (15°C to 25°C). This takes approximately 15 to 20 minutes.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
CAMCEVI Composition
- The active ingredient is leuprolide. A pre-filled syringe with prolonged-release injectable suspension contains leuprolide mesylate, equivalent to 42 mg of leuprolide.
- The other components are poly (D,L-lactide) and N-methylpyrrolidone.
Product Appearance and Container Contents
CAMCEVI is a prolonged-release injectable suspension. The pre-filled syringe contains a viscous and opalescent suspension with a white to pale yellow color.
CAMCEVI is available in containers that contain:
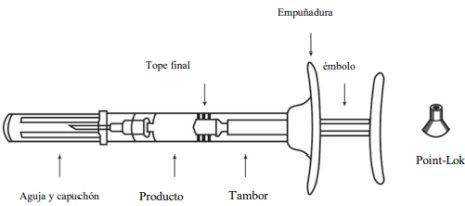
1 pre-filled syringe, 1 needle, and 1 Point-Lok device to protect the needle.
Marketing Authorization Holder
Accord Healthcare S.L.U.
World Trade Center,
Moll de Barcelona, s/n,
Edifici Est 6ª planta,
08039, Barcelona,
Spain
Manufacturer
Accord Healthcare Polska Sp. z.o.o.
Ul. Lutomierska 50
95-200, Pabianice
Poland
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
This information is intended solely for healthcare professionals:
Follow the instructions carefully to ensure the correct preparation of CAMCEVI before administration.
Important:before using it, let CAMCEVI reach room temperature (15 °C to 25 °C). It is recommended to wear gloves during administration.
CAMCEVI contains:
- A blister pack with:
- A sterile pre-filled syringe;
- A sterile needle.
- A non-sterile Point-Lok device to protect the needle.
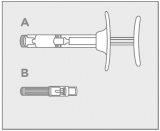
Assembled pre-filled syringe, including the Point-Lok device:
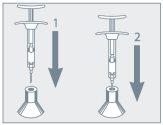
Step 1: Medication Preparation
| Let it reach room temperature and inspect the contents
Do not use the medication if the expiration date has passed.
|
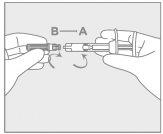
Step 2: Syringe Assembly
Attach the needle
|
|
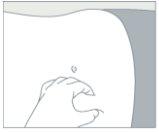
Step 3: Administration Procedure
Prepare the injection site
|
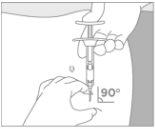
Intra-arterial or intravenous injection should be strictly avoided. |
Administer the treatment
|
Step 4: Dispose of the Needle and Pre-filled Syringe
Needle protection
|
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CAMCEVI 42 mg PROLONGED-RELEASE INJECTABLE SUSPENSIONDosage form: INJECTABLE, 45 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription requiredDosage form: INJECTABLE, 22.5 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription requiredDosage form: IMPLANT, 5 mgActive substance: leuprorelinManufacturer: Sandoz Farmaceutica S.A.Prescription required
Online doctors for CAMCEVI 42 mg PROLONGED-RELEASE INJECTABLE SUSPENSION
Discuss questions about CAMCEVI 42 mg PROLONGED-RELEASE INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions