
Librexa
Ask a doctor about a prescription for Librexa

How to use Librexa
PATIENT INFORMATION LEAFLET
Leaflet accompanying the packaging: patient information
LIBREXA, 11.25 mg, implant in a prefilled syringe
Leuprorelin
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is LIBREXA and what is it used for
- 2. Important information before using LIBREXA
- 3. How to use LIBREXA
- 4. Possible side effects
- 5. How to store LIBREXA
- 6. Contents of the pack and other information
1. What is LIBREXA and what is it used for
The active substance of LIBREXA, leuprorelin acetate, belongs to a group of medicines that reduce the level of certain sex hormones.
LIBREXA works on the pituitary gland, initially stimulating and then inhibiting the production of hormones that regulate the production of male sex hormones in the testes.
This means that the level of sex hormones decreases and remains at the same level during treatment. After stopping LIBREXA, the levels of hormones produced by the pituitary gland and sex hormones return to normal.
LIBREXA is used in men:
- for the symptomatic treatment of advanced prostate cancer that is dependent on hormone activity (prostate cancer);
- as adjunctive treatment during and after radiotherapy, in the treatment of locally advanced, hormone-dependent prostate cancer.
2. Important information before using LIBREXA
When not to use LIBREXA:
- if you are allergic to leuprorelin or any of the other ingredients of this medicine (listed in section 6);
- if the cancer diagnosed in you is not dependent on hormone activity;
- the medicinal product is not intended for use in women, especially during pregnancy and breastfeeding.
Warnings and precautions
Severe skin reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), have been reported with the use of leuprorelin. If you notice any of the symptoms associated with severe skin reactions described in section 4, you should stop using leuprorelin and contact your doctor immediately.
Before starting treatment with LIBREXA, discuss with your doctor:
- if you have high blood pressure. In this case, your doctor will monitor your condition closely.
- if you have a fatty liver
There have been reports of depression in patients taking leuprorelin. If you experience depressive moods while taking LIBREXA, you should inform your doctor.
if you have any of the following conditions: any heart or blood vessel disease, including arrhythmia, or if you are taking medicines for these conditions, you should inform your doctor. The risk of arrhythmias may increase during treatment with LIBREXA.
if you have had both testicles removed. In this case, LIBREXA will not cause further reduction in the level of male sex hormone in the blood.
if you had neurological symptoms (spinal cord compression, metastases to the spine) or discomfort while urinating due to changes in the urinary tract before starting treatment. You should immediately tell your doctor, who will closely monitor your condition in the first weeks of treatment in a hospital setting, if possible.
if the symptoms of the disease recur (e.g., pain, difficulty urinating, or leg weakness during long-term treatment with LIBREXA). In this case, your doctor will regularly check the effectiveness of the treatment by performing appropriate tests (digital rectal examination, imaging tests) and monitoring blood parameters (acid phosphatase activity and PSA levels, as well as testosterone levels).
if you have an increased risk of bone density loss (osteoporosis).
if you have diabetes. In this case, your doctor will monitor your condition very closely.
if you experience severe or recurring headaches, vision problems, and ringing or buzzing in the ears, you should contact your doctor immediately.
Consequences of improper use for doping purposes
The use of LIBREXA may result in positive results in anti-doping tests.
LIBREXA and other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and about medicines you plan to take.
LIBREXA may interact with some medicines used to treat arrhythmias (such as quinidine, procainamide, amiodarone, and sotalol) or increase the risk of arrhythmias when used with certain other medicines, such as methadone (used as a pain reliever and in the detoxification of drug addicts), moxifloxacin (an antibiotic), and antipsychotic medicines (used to treat severe mental disorders).
Pregnancy, breastfeeding, and fertility
LIBREXA is not intended for use in women and should not be used in pregnant or breastfeeding women (see section 2 "When not to use LIBREXA").
Driving and using machines
Fatigue is a common side effect that occurs especially at the beginning of treatment and may also be related to the underlying cancer. Until more information is available, the following precautions should be taken: The medicine, even when used as directed, may affect your reactions to the extent that your ability to drive or operate machinery is impaired. This is more likely to happen when alcohol is consumed during treatment.
3. How to use LIBREXA
LIBREXA should only be administered by a doctor or nurse.
The recommended dose is:
LIBREXA is injected under the skin of the abdomen once every three months.
Treatment of advanced, hormone-dependent prostate cancer with LIBREXA is usually long-term treatment.
LIBREXA must not be administered intravenously (into blood vessels carrying oxygen-rich blood).
Do not stop treatment without consulting your doctor first.
Using a higher dose of LIBREXA than recommended
The medicine is administered by a doctor, so it is unlikely that a higher dose of the medicine will be administered.
Even in the case of a dose of 20 mg of leuprorelin acetate per day for two years, no signs of overdose were observed.
If you have any doubts about the use of this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, LIBREXA can cause side effects, although not everybody gets them.
Frequency not known(frequency cannot be estimated from the available data):
If you notice any of the following symptoms, contact your doctor immediately:
- Red, flat, round, or oval patches on the torso, often with blisters in the center, peeling skin, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin reactions may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis)
- Redness of the skin and itchy rash (toxic skin eruptions)
- Skin reaction causing the appearance of red spots or patches on the skin, which may look like a target with a dark red center surrounded by lighter red rings (erythema multiforme)
Other side effects
Initially, patients usually observe an increase in the level of male sex hormone (testosterone) in the blood, which may cause a temporary worsening of symptoms related to the disease, such as:
bone pain
difficulty urinating due to narrowing of the urinary tract
spinal cord compression
leg weakness
swelling of tissues due to fluid accumulation (edema), also known as lymphedema
The worsening of these symptoms usually resolves on its own without the need to stop using LIBREXA.
At the beginning of therapy, your doctor may prescribe an anti-androgen to alleviate possible disorders related to the initial increase in male sex hormone levels.
During treatment, the level of testosterone decreases to very low values. As a result, some patients may experience the following side effects:
Very common(may affect more than 1 in 10 people):
- Hot flashes
- Decreased or lost sexual desire and potency
- Decreased testicle size
- Bone pain
- Excessive sweating
- Reactions at the injection site, such as redness, pain, swelling, itching, which usually resolve during treatment
- Weight gain
Common(may affect up to 1 in 10 people):
- Breast enlargement in men
- Increased appetite
- Decreased appetite
- Mood swings
- Depression
- Sleep disorders
- Headache
- Abnormal sensations (paresthesia) / numbness
- Nausea, vomiting
- Joint and / or back pain
- Muscle weakness
- Increased frequency of urination at night
- Urination problems
- Frequent need to urinate small amounts
- Fatigue
- Fluid accumulation in tissues (edema)
- Weight loss
- Increased activity of liver enzymes (ALT, AST, gamma-GT) and other enzymes (LDH, alkaline phosphatase), which may also be related to the disease.
Uncommon(may affect up to 1 in 100 people):
- Generalized allergic reactions (fever, itching, increased white blood cell count [eosinophilia], skin rash)
- Diarrhea
- Dryness of the skin and / or mucous membranes
- Increased sweating at night
- Inability to completely empty the bladder
- Testicular pain
Rare(may affect up to 1 in 1,000 people):
- Decreased or increased blood sugar levels
- Dizziness
- Transient taste disturbances
- Changes in blood pressure (decreased or increased blood pressure)
- Hair loss
Very rare(may affect up to 1 in 10,000 people):
- Severe allergic reactions (anaphylactic reactions)
Seek medical attention immediatelyif you experience any of the following symptoms of a severe allergic reaction, which can very rarely occur with the use of LIBREXA and requires immediate medical attention:
redness with swelling of the skin and mucous membranes, difficulty breathing due to narrowing of the airways, decreased blood pressure, rapid heartbeat, seizures, and in severe cases: life-threatening circulatory failure.
- As with other medicines in this class: pituitary apoplexy after the first administration of the medicine in patients with pituitary tumors.
Frequency not known(frequency cannot be estimated from the available data):
- Pneumonia, lung disease
- Changes in ECG (prolonged QT interval)
- Seizures
- Idiopathic intracranial hypertension (increased intracranial pressure around the brain, characterized by headache, double vision, and other vision problems, as well as ringing or buzzing in one or both ears)
Special information:
The response to LIBREXA treatment should be monitored by measuring the level of male sex hormone (testosterone) in the blood 28 days after each injection and before each subsequent administration of LIBREXA, as well as on the basis of other laboratory tests, such as measurement of acid phosphatase activity and PSA levels. For example, in the initial phase of treatment, the level of testosterone first increases and then decreases within 2 weeks. After 2 to 4 weeks, testosterone levels are comparable to those observed after bilateral orchiectomy and remain stable throughout the treatment period.
In the initial phase of treatment, a transient increase in acid phosphatase activity (detected in laboratory tests) may occur. Return to physiological or almost physiological values occurs after several weeks.
The decrease in sex hormone levels (testosterone) that occurs after orchiectomy or as a result of the use of medicines that suppress sex hormones (such as LIBREXA) may cause a decrease in bone density with a higher risk of fractures (see section 2 "Warnings and precautions"). The decrease in bone density is more noticeable after orchiectomy than after administration of LIBREXA.
An abscess at the injection site is a rare complication. A case of abscess at the injection site has been reported, which was associated with reduced absorption of leuprorelin. In such cases, it is recommended to determine the level of testosterone.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store LIBREXA
Do not use this medicine after the expiry date stated on the carton and blister pack after EXP.
The prefilled syringe should be used immediately after removal from the blister pack.
Do not store above 30°C.
Store the prefilled syringe in the original package to protect from moisture.
Keep the medicine out of the sight and reach of children.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What LIBREXA contains:
The active substance of LIBREXA is leuprorelin acetate.
1 implant contains: 10.72 mg leuprorelin (Leuprorelinum), which corresponds to 11.25 mg leuprorelin acetate
The other ingredients are: DL-lactide and glycolide copolymer (1:1) and DL-lactide polymer.
What LIBREXA looks like and contents of the pack
A prefilled syringe made of MMBS polymer (Methyl Methacrylate Butadiene Styrene), with a plunger and needle made of stainless steel, placed in a pouch made of PETP/Aluminum/PE film, containing a desiccant, in a cardboard box.
The pack contains:
1 prefilled syringe with 1 implant for subcutaneous injection.
Marketing authorization holder
LEK-AM Pharmaceutical Company Ltd.
Ostrzykowizna 14A
05-170 Zakroczym
Poland
Manufacturer
AMW GmbH
Birkerfeld 11
83627 Warngau,
Germany
Date of last revision of the leaflet: 31.12.2024
Information intended for healthcare professionals only:
Instructions for use
Image 1
Remove the applicator from the sterile packaging
Check if the implant is visible inside the applicator in the correct position
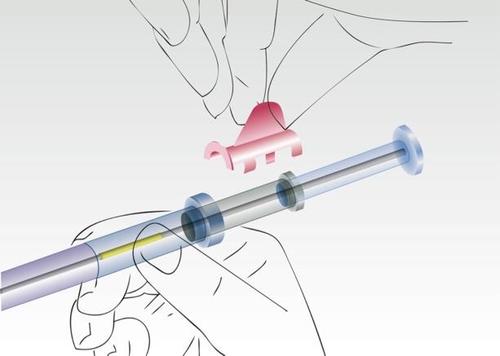
Remove the safety ring.
Image 2
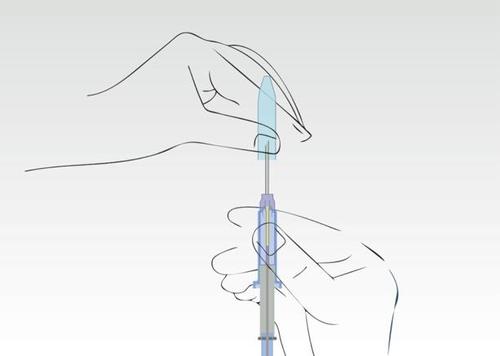
Hold the applicator on the cylinder of the syringe and remove the nozzle.
Image 3
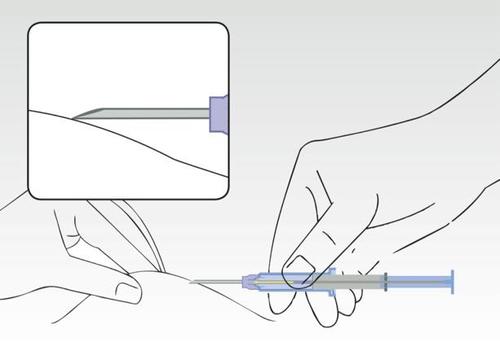
Simultaneously squeeze the patient's skin and, holding the cylinder of the syringe, insert the needle at an angle (almost parallel to the skin) with the needle tip pointing upwards.
Insert the needle into the subcutaneous tissue (not into the muscle or abdominal cavity) of the anterior abdominal wall below the navel, until the cylinder of the syringe touches the patient's skin.
The cylinder of the syringe must remain in contact with the skin during the entire application process!
Image 4
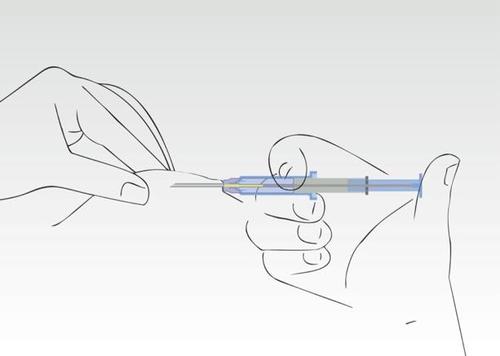
Press the plunger of the syringe. The implant moves to the end of the needle.
Do not pull the syringe back. During the application process, the cylinder of the syringe must touch the patient's skin.
Image 5
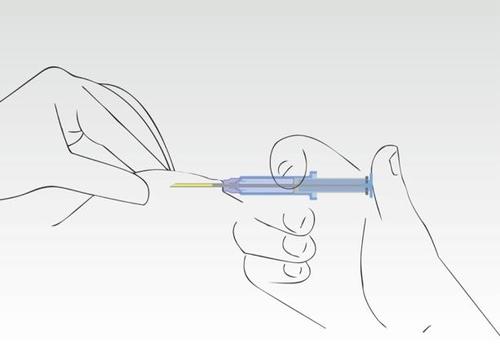
When the plunger is pressed, the needle retracts automatically.
Image 6
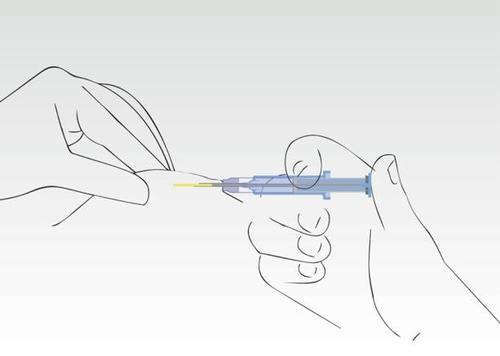
The needle retracts into the cylinder of the syringe. The cylinder of the syringe must remain in contact
with the patient's skin.Usually, pressing the plunger and retracting the needle occurs in one smooth motion.
Image 7
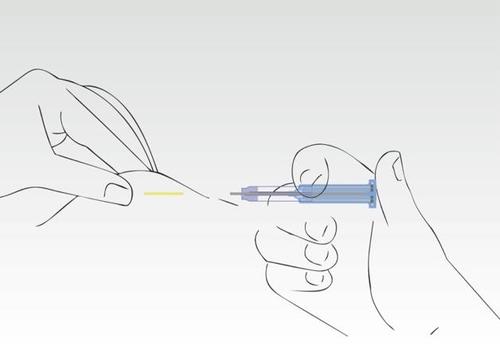
The application process is complete. The needle has been fully retracted into the cylinder of the syringe.
The supernatant protects against injury to the needle tip.
Image 8
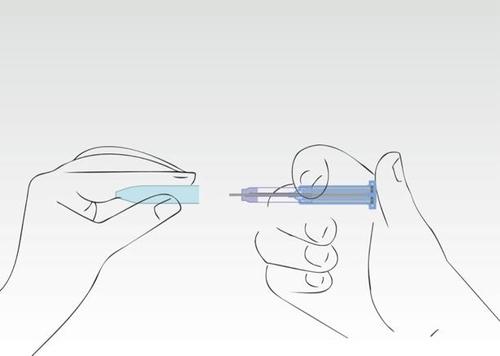
Put the nozzle back on.
The syringe should be disposed of in a designated container.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterAMW GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LibrexaDosage form: Powder, 22.5 mgActive substance: leuprorelinPrescription requiredDosage form: Powder, 45 mgActive substance: leuprorelinPrescription requiredDosage form: Powder, 7.5 mgActive substance: leuprorelinPrescription required
Alternatives to Librexa in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Librexa in Spain
Alternative to Librexa in Ukraine
Online doctors for Librexa
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Librexa – subject to medical assessment and local rules.











