ELIGARD SEMESTRAL 45 mg POWDER AND DILUENT FOR INJECTION

How to use ELIGARD SEMESTRAL 45 mg POWDER AND DILUENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Eligard Semestral 45 mg
Powder and Solvent for Solution for Injection
Leuprorelin, acetate.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the Package Leaflet:
- What is Eligard Semestral and what is it used for
- What you need to know before you use Eligard Semestral
- How to use Eligard Semestral
- Possible side effects
- Storage of Eligard Semestral
- Contents of the pack and other information
1. What is Eligard Semestral and what is it used for
The active substance of Eligard Semestral belongs to the group of gonadotropin-releasing hormone analogues. These medicines are used to reduce the production of certain sex hormones (testosterone).
Eligard Semestral is used to treat hormone-dependent metastatic prostate cancerin adult men and in the treatment of high-risk non-metastatic hormone-dependent prostate cancer in combination with radiotherapy.
2. What you need to know before you use Eligard Semestral
Do not use Eligard Semestral
- If you are a woman or a child.
- If you are hypersensitive (allergic)to the active substance leuprorelin acetate, to medicines with a similar activity to natural gonadotropin-releasing hormone, or to any of the other components of Eligard Semestral (listed in section 6).
- After surgical removal of your testicles, as in this case, Eligard Semestral will not lead to a further decrease in serum testosterone concentrations.
- As sole treatment if you are experiencing symptoms related to pressure on the spinal cord or with a tumor in the spinal column. In this case, Eligard Semestral may only be used in combination with other medicines for prostate cancer.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting treatment with Eligard Semestral
- If you have: any heart or blood vessel disorders, including heart rhythm problems (arrhythmias), or if you are being treated with medicines to correct these disorders. The risk of heart rhythm problems may be increased with the use of Eligard Semestral.
- If you have difficulty urinating. You should be closely monitored during the first weeks of treatment.
- If you start to experience pressure on the spinal cord or difficulty urinating. As serious cases (related to other medicines with a similar mechanism of action to Eligard Semestral) of pressure on the spinal cord and narrowing of the ducts between the kidneys and the bladder that can contribute to the appearance of similar symptoms to paralysis have been reported. If these complications occur, the usual treatment for these cases should be initiated.
- If you experience, within two weeks of taking Eligard Semestral, sudden severe headache, vomiting, altered mental status, and sometimes heart collapse, inform your doctor or medical team. Rarely, these cases have been reported with OTHER MEDICINES that have a similar mechanism to Eligard Semestral, and are known as pituitary apoplexy.
- If you suffer from diabetes mellitus(high blood sugar levels). You should be frequently monitored during treatment.
- Treatment with Eligard Semestral may increase the risk of fractures due to osteoporosis (decrease in bone density).
- Cases of depression have been reported in patients using Eligard Semestral. If you are using Eligard Semestral and start to experience a depressed mood, inform your doctor.
- Cases of cardiovascular effects have been reported in patients using similar medicines to Eligard Semestral, which are not known to be related to these medicines. If you are using Eligard Semestral and start to experience cardiovascular signs or symptoms, inform your doctor.
- Cases of epileptic seizures have been reported in patients after administration of Eligard Semestral. If you are using Eligard Semestral and start to experience epileptic seizures, inform your doctor.
- Contact your doctor immediately if you experience severe or recurrent headaches, vision problems, or ringing in the ears.
- You have fatty liver.
Severe skin reactions, including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), have been reported in association with leuprorelin. Discontinue the use of leuprorelin and seek medical attention immediately if you notice any symptoms related to these severe skin reactions described in section 4.
Complications at the start of treatment
During the first week of treatment, there is usually a brief increase in the male sex hormone, testosterone, in the blood. This may lead to a temporary worseningof symptoms related to the disease and also to the appearance of new symptoms that had not been experienced until then. These symptoms include especially bone pain, urinary disorders, pressure on the spinal cord, or blood in the urine. These symptoms usually resolve with continued treatment. If the symptoms do not resolve, you should contact your doctor.
If you do not improve with Eligard Semestral
A proportion of patients will have tumors that are not sensitive to the decrease in serum testosterone concentrations. If you feel that the effect of Eligard Semestral is not as expected, inform your doctor.
Use of Eligard Semestral with other medicines
This medicine may interfere with some medicines used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with certain medicines (e.g., methadone (used for pain relief and as part of drug detoxification), moxifloxacin (an antibiotic), antipsychotics used for severe mental illnesses).
Inform your doctor or pharmacist if you are using or have recently used any other medicines, including those obtained without a prescription.
Pregnancy and breastfeeding
This medicine is contraindicated in women.
Driving and using machines
Fatigue, dizziness, and vision disturbances are possible side effects of treatment with Eligard Semestral or may appear due to the disease. If you experience these side effects, be careful when driving or using machines.
3. How to use Eligard Semestral
Dose
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Unless your doctor prescribes otherwise, this medicine is administered onceevery six months.
The injectable solution forms a deposit of the active substance from which there is a continuous release of the active substance, leuprorelin acetate, over a period of six months.
Additional tests
The response to treatment with this medicine should be reviewed by your doctor by checking specific clinical values and determining the blood concentrations of the so-called prostate-specific antigen (PSA).
Method of administration
This medicine should only be administered by your doctoror nurse. They will be responsible for its preparation.
After preparation, Eligard Semestral is administered as a subcutaneous injection (injection into the tissue under the skin). Intra-arterial (into an artery) or intravenous (into a vein) injection should be totally avoided. As with other active substances that are injected subcutaneously, the injection site should be changed periodically.
If you receive more Eligard Semestral than you should
Since the injection is usually administered by your doctor or qualified personnel, it is not expected that an overdose will occur.
If, however, a larger amount than planned has been administered, contact your doctor so that they can monitor you especially and administer additional treatment as necessary or consult the Toxicology Information Service. Telephone 91 562 04 20.
If you forget to use Eligard Semestral
Talk to your doctor if you think you have forgotten your semi-annual administration of the medicine.
Effects when stopping treatment with Eligard Semestral
As a general rule, treatment of prostate cancer with this medicine is prolonged. Therefore, treatment should not be interrupted even if there is an improvement in symptoms or if they disappear completely.
If treatment with this medicine is interrupted prematurely, it may lead to a worsening of symptoms related to the disease.
Do not interrupt treatment prematurely without consulting your doctor first.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Eligard Semestral can cause side effects, although not everybody gets them.
The side effects that have been observed during treatment with Eligard Semestral are mainly attributed to the specific effect of the active substance, leuprorelin acetate, particularly the increase and decrease of certain hormones. The most frequently described side effects are hot flashes (approximately 58% of patients), nausea, general malaise, and fatigue, as well as temporary local irritation at the injection site.
Side effects at the start of treatment
During the first weeks of treatment with Eligard Semestral, the specific symptoms of the disease may worsen because there is a brief increase in the male sex hormone, testosterone, in the blood. Therefore, your doctor may administer an appropriate anti-androgen (substance that inhibits the effect of testosterone) in the initial phase of treatment to reduce possible undesirable effects. (See also Section 2Before using Eligard Semestral, Complications at the start of treatment).
Local side effects
The local side effects that have been described after injection of Eligard Semestral are typically those that are often associated with similar preparations that are injected subcutaneously (preparations that are injected into the tissue under the skin). Mild burning immediately after injection is very common. Stinging and pain after injection, as well as bruising at the injection site, are common. Redness of the skin at the injection site has been frequently reported. Hardening of the tissues and ulceration are uncommon.
These local side effects after subcutaneous injection are mild and have been described as being of short duration. They do not recur between individual injections.
Very common side effects (may affect more than 1 in 10 people)
- Hot flashes
- Spontaneous bleeding in the skin or mucous membrane, redness of the skin
- Fatigue, injection-related side effects (See also local side effects above)
Common side effects (may affect up to 1 in 10 people)
- Nasopharyngitis (common cold symptoms)
- Nausea, general malaise, diarrhea, inflammation of the stomach and intestines (gastroenteritis/colitis)
- Itching, night sweats
- Pain in the joints
- Urination irregularly (also at night), difficulty starting to urinate, pain when urinating, reduced urine volume
- Breast tenderness, breast inflammation, decreased testicle size, testicular pain, infertility, erectile dysfunction, reduced penis size
- Stiffness (episodes of exaggerated trembling with high fever), weakness
- Prolonged bleeding time, changes in blood values, decreased red blood cell count
Uncommon side effects (may affect up to 1 in 100 people)
- Urinary tract infection, local skin infection
- Worsening of diabetes mellitus
- Abnormal dreams, depression, decreased libido
- Dizziness, headache, skin sensitivity disorders, insomnia, taste disorders, smell disorders
- Hypertension (high blood pressure), hypotension (low blood pressure)
- Shortness of breath
- Constipation, dry mouth, dyspepsia (indigestion, symptoms of having a full stomach, stomach pain, belching, nausea, vomiting, sensation of heartburn in the stomach), vomiting
- Flushes, increased sweating
- Lower back pain, muscle cramps
- Hematuria (blood in urine)
- Bladder spasms, frequent urination, inability to urinate
- Male breast tissue growth, impotence
- Lethargy (drowsiness), pain, fever
- Weight gain
- Loss of balance, dizziness
- Muscle mass loss/muscle tissue loss after prolonged use
Rare side effects (may affect up to 1 in 1,000 people)
- Abnormal involuntary movements
- Sudden loss of consciousness, fainting
- Flatulence, belching
- Hair loss, skin rash (pimples on the skin)
- Chest pain
- Ulceration at the injection site
Very rare side effects (may affect up to 1 in 10,000 people)
- Necrosis at the injection site
Not known (frequency cannot be estimated from the available data)
- Changes in the electrocardiogram (QT interval prolongation)
- Lung inflammation, lung disease
- Idiopathic intracranial hypertension (increased pressure around the brain characterized by headaches, double vision, and other visual symptoms, ringing in the ears)
- If you notice circular or target-shaped, reddish-colored patches on your trunk, often with central blisters, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin reactions may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome/toxic epidermal necrolysis)
- Redness of the skin and rash with itching (Toxic skin rash)
- A skin reaction that causes red or target-shaped spots on the skin (Erythema multiforme)
Other side effects
Other side effects published in relation to treatment with leuprorelin, the active substance of Eligard Semestral, are edema (fluid accumulation in tissue, appearing as swelling of hands and feet), pulmonary embolism (produces symptoms such as shortness of breath, difficulty breathing, and chest pain), palpitations (perception of heartbeats), muscle weakness, chills, skin rash, memory loss, and vision impairment. An increase in bone tissue loss (osteoporosis) can be expected after prolonged treatment with Eligard Semestral. Due to osteoporosis, the risk of fractures increases.
Severe allergic reactions that cause difficulty breathing or dizziness after administration of products of the same class as Eligard Semestral have been rarely reported.
Epileptic seizures have been reported after administration of products of the same class as Eligard Semestral.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Eligard Semestral
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
Storage instructions
Store in a refrigerator (between 2°C and 8°C).
Store in the original packaging to protect from moisture.
This product should be at room temperature before injection. Remove from the refrigerator approximately 30 minutes before use. Once removed from the refrigerator, this product can be stored in its original packaging at room temperature (below 25°C) for up to 4 weeks.
Once the tray is opened, the product should be prepared immediately and used immediately. For single use only.
Instructions for disposal of unused or expired Eligard Semestral packaging
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Eligard Semestral
The active ingredient is leuprolide acetate.
A preloaded syringe (syringe B) contains 45 mg of leuprolide acetate.
The other components are poly (DL-lactic-co-glycolic) acid (85:15) and N-methyl-2-pyrrolidone in the preloaded syringe with injectable solution (syringe A).
Appearance of Eligard Semestral and Container Contents
Powder and solvent for injectable solution.
It is available in the following containers:
- A thermoformed tray-type container and a sterile 18-gauge needle inserted into a cardboard support. The tray contains a bag with a desiccant and a preconnected syringe system consisting of:
- syringe A preloaded with the solvent
- syringe B preloaded with the powder and a connector with a snap-on button for syringe A and B
- A multiple container that contains 2 preconnected syringe system kits.
Only some container sizes may be marketed.
Marketing Authorization Holder
Recordati Industria Chimica e Farmaceutica S.p.A.
Via Matteo Civitali, 1
20148 Milan
Italy
Manufacturer
Recordati Industria Chimica e Farmaceutica S.p.A.
Via Matteo Civitali, 1
20148 Milan
Italy
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Casen Recordati, S.L.
Autovía de Logroño, km 13,300
50180 Utebo - Zaragoza
Spain
This medication is authorized in the member states of the European Economic Area under the following names:
Austria: Eligard Depot 45 mg
Belgium: Depo-Eligard 45 mg
Bulgaria: Eligard 45 mg
Cyprus: Eligard
Czech Republic: Eligard
Denmark: Eligard
Estonia: Eligard
Finland: Eligard
France: Eligard 45 mg
Germany: Eligard 45 mg
Hungary: Eligard 45 mg
Iceland: Eligard
Ireland: Eligard 45 mg
Italy: Eligard
Latvia: Eligard 45 mg
Lithuania: Eligard 45 mg
Luxembourg: Depo-Eligard 45 mg
Netherlands: Eligard 45 mg
Norway: Eligard
Poland: Eligard 45 mg
Portugal: Eligard 45 mg
Romania: Eligard 45 mg
Slovakia: Eligard 45 mg
Slovenia: Eligard 45 mg
Spain: Eligard Semestral 45 mg
Sweden: Eligard
Date of the last revision of this prospectus: 10/2024
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
This information is intended only for healthcare professionals:
Wait for the medication to reach room temperature by removing it from the refrigerator approximately 30 minutes before use.
Please first prepare the patient for the injection, then prepare the medication by following the instructions indicated below. If the medication is not prepared using the correct technique, it should not be administered, as clinical efficacy may be lacking due to its incorrect reconstitution.
Step 1
In a clean area, open the tray by removing the aluminum from the corner to remove the contents. Discard the desiccant bag. Remove the preconnected syringe system (Figure 1.1) from the tray. Open the safety needle package (Figure 1.2) by removing the paper tab Note:syringe A and syringe B should not be aligned yet.
Figure 1.1 Tray contents: preconnected syringe system | Figure 1.2 Under the tray: safety needle and cap |
|
|
Step 2
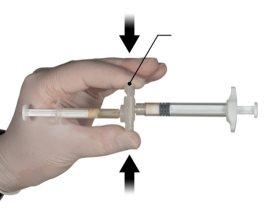
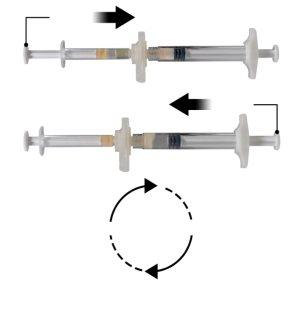
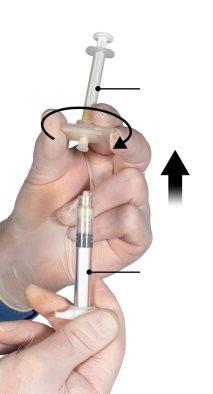
Hold the snap-on button of the connector with your index finger and thumb and press (Figure 2) until you hear a click. The two syringes will be aligned. No particular orientation of the syringe system is required to activate the connector. Do not bend the syringe system (please note that some medication may leak out if the syringes are partially unscrewed).
Figure 2 |
|
Step 3
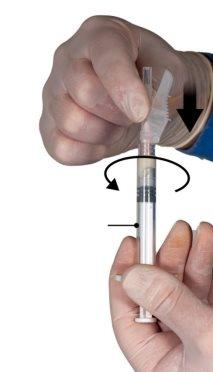
Hold the syringes in a horizontal position, transfer the liquid contents of syringe A to the leuprolide acetate powder in syringe B. Mix the product well for 60 cycles by gently pressing the contents of both syringes back and forth between the syringes (one cycle is a plunger push for syringe A and a plunger push for syringe B) in a horizontal position to obtain a homogeneous and viscous solution (Figure 3). Do not bend the syringe system (please note that some medication may leak out if the syringes are partially unscrewed).
Figure 3 |
|
When thoroughly mixed, the viscous solution will have a color within the range of colorless to pale yellow (which may include shades from white to pale yellow).
Important: after mixing, proceed immediately to the next step, as the viscosity of the product increases over time. Do not refrigerate the medication once reconstituted.
Please note: The medication must be mixed as described; shaking will not produce an adequate mixture.
Step 4
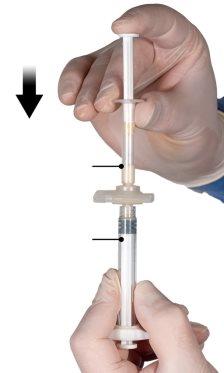
After mixing, hold the syringes vertically with syringe B at the bottom. The syringes must remain well connected. Transfer all the contents to syringe B (wide syringe) by pressing the plunger of syringe A and slightly retracting the plunger of syringe B (Figure 4).
Figure 4 |
|
Step 5
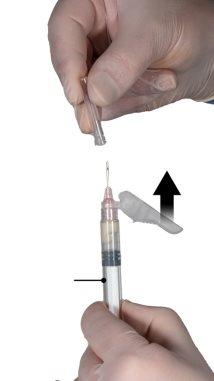
While ensuring that the plunger of syringe A is completely pressed down, hold the connector and unscrew it from syringe B. Syringe A will remain attached to the connector (Figure 5). Make sure that no contents leak out, as the needle will not fit properly when attached.
Please note: During preparation, a large air bubble or several small ones may remain - this is acceptable. Do not purge the air bubbles from syringe B at this time, as medication will be lost!
Figure 5 |
|
Step 6
- Hold syringe B vertically and keep the white plunger back to avoid losing medication.
- Attach the safety needle to syringe B by holding the syringe and gently turning the needle clockwise, approximately three-quarters of a turn, until the needle is secured (Figure 6).
Do not overtighten, as the needle cone may crack and medication may leak during injection. The safety cap may also be damaged if the needle is screwed on too tightly.
The medication should not be used if the needle cone is cracked, damaged, or if the contents leak. The damaged needle should not be replaced, and the medication should not be injected. All elements of the administration device should be discarded safely.
If the needle cone is damaged, the medication should be replaced with a new one.
Figure 6 |
|
Step 7
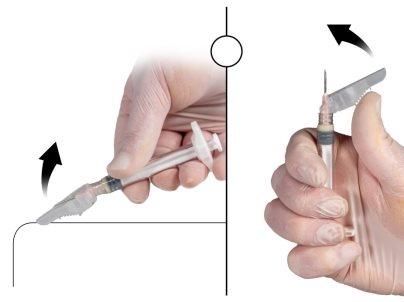
Immediately before administration, remove the safety cap from the needle and remove the protective cap from the needle (Figure 7).
Important: Do not manipulate the safety needle mechanism before administration. If the needle appears to be damaged or has leaks, the product should NOT be used. The damaged needle should NOT be replaced, and the medication should NOT be injected. In case of needle damage, use another ELIGARD kit.
Figure 7 |
|
Step 8
Before administration, purge any largeair bubbles from syringe B. Administer the medication subcutaneously while keeping the safety cap away from the needle.
Administration procedure:
- Select an injection site on the abdomen, upper buttocks, or other area with adequate subcutaneous tissue that does not have excess pigmentation, nodules, lesions, or hair and has not been used recently.
- Clean the injection area with an alcohol swab (not included).
- Using your thumb and index finger, pinch and gather the skin around the injection site.
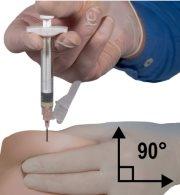
- Using your dominant hand, insert the needle quickly at a 90° angle to the skin surface. The depth of penetration will depend on the amount and width of subcutaneous tissue and the length of the needle. After inserting the needle, release the skin.
- Inject the medication by slowly and constantly pushing the plunger until the syringe is empty. Make sure to inject the total amount of medication from syringe B before removing the needle.
- Remove the needle quickly at the same 90° angle used for insertion while maintaining pressure on the plunger.
Figure 8

Step 9
After injection, block the safety mechanism using one of the activation methods mentioned below.
- Closing on a flat surface
Press the safety cap down with the slider against a flat surface (Figure 9a) to cover the needle and block the cap.
Verify the locked position with an audible and tactile "click". The locked position will completely cover the needle tip.
- Closing with your thumb
Place your thumb on the safety cap (Figure 9b) to cover the needle tip and block the cap.
Verify the locked position with an audible and tactile "click". The locked position will completely cover the needle tip.
Figure 9a Closing on a flat surface | Figure 9b Closing with your thumb |
|
Once the safety cap is locked, immediately discard the needle and syringe in an authorized container for sharp objects.
- Country of registration
- Average pharmacy price549.08 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ELIGARD SEMESTRAL 45 mg POWDER AND DILUENT FOR INJECTIONDosage form: INJECTABLE, 42 mgActive substance: leuprorelinManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 22.5 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription requiredDosage form: IMPLANT, 5 mgActive substance: leuprorelinManufacturer: Sandoz Farmaceutica S.A.Prescription required
Online doctors for ELIGARD SEMESTRAL 45 mg POWDER AND DILUENT FOR INJECTION
Discuss questions about ELIGARD SEMESTRAL 45 mg POWDER AND DILUENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions