
Eligard 45 mg

Ask a doctor about a prescription for Eligard 45 mg

How to use Eligard 45 mg
Leaflet attached to the packaging: information for the user
Eligard 45 mg,
powder and solvent for solution for injection
Leuprolide acetate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Eligard and what is it used for
- 2. Important information before using Eligard
- 3. How to use Eligard
- 4. Possible side effects
- 5. How to store Eligard
- 6. Contents of the pack and other information
1. What is Eligard and what is it used for
The active substance of Eligard belongs to a group of medicines called gonadorelin analogues. These medicines are used to inhibit the production of certain sex hormones (testosterone).
Eligard is used in adult men for the treatment of prostate cancerwith metastases, sensitive to hormone therapy, and in combination with radiotherapy - for the treatment of high-risk prostate cancer without metastases, sensitive to hormone therapy.
2. Important information before using Eligard
When not to use Eligard
- In women and children.
- If the patient is allergicto the active substance - leuprolide acetate, to any similar-acting compound - gonadotropin or to any of the other ingredients of Eligard (listed in section 6).
- After surgical removal of the testes, because Eligard does not cause further reduction in serum testosterone levels.
- As the only treatment, if the patient has symptoms related to spinal cord compression or metastases to the spine. In such cases, Eligard can only be used in combination with other medicines used to treat prostate cancer.
Warnings and precautions
Before starting treatment with Eligard, discuss it with your doctor, pharmacist, or nurse:
- If you have any of the following conditions: any heart or blood vessel disease, including rhythm disorders (arrhythmia) or if you are taking medicines for these diseases. The risk of rhythm disorders may increase during treatment with Eligard.
- If you have difficulty urinating. In such cases, the patient's condition should be monitored during the first weeks of treatment.
- If spinal cord compression or difficulty urinating worsens. During concurrent use of similar-acting medicines, severe cases of spinal cord compression and ureteral narrowing have been observed, resulting in symptoms such as paralysis. In such cases, standard treatment is necessary.
- If the patient experiences sudden headache, vomiting, change in mental status, or circulatory collapse within two weeks of Eligard administration. In such cases, the doctor or medical staff should be notified immediately. These are symptoms of a rare condition called pituitary apoplexy, which has been reported in association with the use of OTHER MEDICINES with similar action to Eligard.
- If the patient has diabetes(high blood sugar levels). In such cases, the patient's condition should be monitored during treatment.
- Treatment with Eligard may increase the risk of fractures due to osteoporosis (decreased bone density).
- There have been reports of depression in patients taking Eligard. If depressive moods occur during Eligard treatment, the doctor should be informed.
- There have been reports of cardiovascular disease in patients taking similar medicines to Eligard - it is not known whether their occurrence is related to the use of these medicines. If symptoms of cardiovascular disease occur during Eligard treatment, the doctor should be informed.
- There have been reports of seizures in patients who received Eligard. If seizures occur during Eligard treatment, the doctor should be informed.
- If the patient experiences severe or recurrent headaches, vision problems, or ringing in the ears, they should immediately consult a doctor.
- If the patient has a fatty liver
In connection with the use of leuprolide, severe skin rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), have been reported. In case of noticing any symptoms related to severe skin reactions described in section 4, the use of leuprolide should be discontinued and a doctor should be contacted immediately.
Complications occurring in the initial period of Eligard use
During the first week of use, a temporary increase in testosterone levels in the blood is observed, which may lead to transient worseningof disease symptoms or the appearance of new ones that have not occurred before. These symptoms include, in particular: bone pain, urinary disorders, spinal cord compression, and the appearance of blood in the urine.
These symptoms usually resolve during continued treatment. If the symptoms do not resolve, the attending doctor should be consulted.
Lack of Eligard effect
In some patients, tumors that are not sensitive to the reduction of testosterone levels in the blood are found. If it is felt that Eligard is not having a sufficient effect, the attending doctor should be informed.
Eligard and other medicines
Eligard may affect the action of some medicines used to treat rhythm disorders (e.g., quinidine, procainamide, amiodarone, and sotalol) or increase the risk of rhythm disorders when used with certain other medicines (e.g., methadone (used to relieve pain and detoxify narcotics), moxifloxacin (an antibiotic), antipsychotic medicines used to treat severe mental illnesses).
Inform your doctor or pharmacist about all medicines you are currently taking or have recently taken, including those that are available without a prescription.
Pregnancy and breastfeeding
Eligard is contraindicated in women.
Driving and using machines
Fatigue, dizziness, and vision disturbances may be side effects of Eligard or result from the underlying disease. If the above-mentioned side effects occur, caution should be exercised when driving vehicles and operating machines.
3. How to use Eligard
Dosage
This medicine should always be used as directed by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist.
If the doctor does not prescribe otherwise, Eligard is administered once every 6 months.
The injected solution forms a reservoir of the active substance, from which the active substance - leuprolide acetate - is continuously released over 6 months.
Additional tests
The response to Eligard treatment should be monitored by the attending doctor based on clinical symptoms and the level of prostate-specific antigen (PSA) in the blood.
Method of administration
Eligard should only be administered by a doctoror nurse, who will also prepare the product.
After preparation, Eligard is administered as a subcutaneous injection (injection into the tissue under the skin). It is absolutely necessary to avoid intravenous (into a vein) or intra-arterial (into an artery) injection. As with other active substances used in subcutaneous injections, the injection sites should be periodically changed.
Use of a higher than recommended dose of Eligard
The medicine is usually administered by a doctor or properly trained medical staff, so it is unlikely that a higher dose of the medicine will be administered.
If a higher dose of the medicine is administered, the attending doctor will recommend monitoring the patient and appropriate treatment if necessary.
Missed administration of Eligard
If it is suspected that a 6-month dose of the medicine has been missed, the attending doctor should be informed.
Discontinuation of Eligard treatment
Treatment of advanced prostate cancer requires long-term administration of Eligard.
Therefore, treatment should not be discontinued even if the patient's condition improves or the disease symptoms disappear.
If treatment is discontinued prematurely, the disease symptoms may worsen.
Do not discontinue treatment without prior consultation with a doctor.
In case of any further doubts about the use of this medicine, consult a doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Eligard can cause side effects, although not everybody gets them.
The side effects observed during Eligard treatment result mainly from the specific action of leuprolide acetate, which increases or decreases the levels of certain hormones. The most commonly observed side effects are hot flashes (in about 58% of patients), nausea, malaise, and fatigue, as well as transient reactions at the injection site.
Side effects in the initial treatment period
During the first weeks of treatment with Eligard, there may be a worsening of disease symptoms due to the initial, temporary increase in the level of the male sex hormone - testosterone in the blood. Therefore, the doctor may recommend taking an appropriate anti-androgen (a substance that reduces the action of testosterone) in the initial phase of treatment to reduce the above-mentioned effect of the medicine (see also section 2. Important information before using Eligard; Complications occurring in the initial period of Eligard use).
Local side effects
Local side effects described after injection of Eligard are those that occur frequently after subcutaneous injection (injection into the tissue under the skin) of similar medicines. Mild burning sensation immediately after injection occurs very frequently. Stinging and pain after injection, as well as bruising at the injection site, occur frequently.
Redness at the injection site has been reported frequently. Hardening of tissues and ulcers at the injection site occur infrequently.
The above-mentioned local side effects occurring after subcutaneous injection are mild and described as short-term. They do not recur between consecutive injections.
Very common side effects (may affect more than 1 in 10 people)
- Hot flashes
- Spontaneous bleeding from the skin and mucous membranes, redness of the skin
- Fatigue, reactions at the injection site (see above:Local side effects)
Common side effects (may affect up to 1 in 10 people)
- Nasopharyngitis (cold symptoms)
- Nausea, malaise, diarrhea, gastritis (stomach and intestine/colon inflammation)
- Itching, night sweats
- Joint pain
- Urinary frequency (including at night), difficulty urinating, painful urination, decreased urine output
- Breast tenderness, breast swelling, testicular atrophy, testicular pain, infertility, erectile dysfunction, decreased penis size
- Chills (episodes of increased shivering with high fever), weakness
- Prolonged bleeding time, changes in blood parameter values, decreased red blood cell count/low red blood cell count
Uncommon side effects (may affect up to 1 in 100 people)
- Urinary tract infections, local skin infections
- Worsening of diabetes symptoms
- Unusual dreams, depression, decreased libido
- Dizziness, headache, skin sensation disorders, insomnia, taste disorders, smell disorders
- Hypertension (high blood pressure), hypotension (low blood pressure)
- Shortness of breath
- Constipation, dry mouth, indigestion (impaired digestion with a feeling of fullness of the stomach, stomach pain, belching, nausea, vomiting, heartburn), vomiting
- Increased skin moisture, excessive sweating
- Back pain, muscle cramps
- Hematuria (blood in the urine)
- Bladder spasm, frequent urination, inability to urinate
- Breast enlargement in men, impotence
- Lethargy (drowsiness), pain, fever
- Weight gain
- Loss of balance, feeling of emptiness in the head
- Muscle atrophy/muscle loss after long-term use
Rare side effects (may affect up to 1 in 1,000 people)
- Involuntary movements
- Sudden loss of consciousness, fainting
- Bloating with gas, belching
- Hair loss, skin rashes (pustules on the skin)
- Chest pain
- Ulceration at the injection site
Very rare side effects (may affect less than 1 in 10,000 people)
- Necrosis at the injection site
Frequency not known (cannot be estimated from the available data)
- Changes in the electrocardiogram (QT interval prolongation)
- Pneumonia, lung disease
- Idiopathic intracranial hypertension (increased intracranial pressure
around the brain, characterized by headache, double vision, and other vision-related symptoms, as well as ringing in one or both ears)
- Red, flat, round, or coin-shaped spots on the torso, often with blisters in the center, peeling skin, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin rashes may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis)
- Redness of the skin and itchy rash (toxic skin eruptions)
- Skin reaction causing the appearance of red spots or patches on the skin, which may look like a target with a dark red center surrounded by lighter red rings (erythema multiforme)
Other side effects
Other side effects described in the literature as associated with leuprolide treatment - the active substance contained in Eligard - include: peripheral edema (fluid accumulation in tissues, manifested as swelling of the hands and feet), pulmonary embolism (manifested as shortness of breath, difficulty breathing, and chest pain), palpitations (perceptible heartbeat), decreased muscle strength, chills, rash, and memory and vision disorders. With long-term treatment with Eligard, an increased frequency of osteoporosis (decreased bone density) symptoms can be expected. Osteoporosis increases the risk of fractures.
Rarely, severe allergic reactions have been reported after the use of products belonging to the same group as Eligard, causing breathing difficulties or dizziness.
Seizures have been reported after the use of products belonging to the same group as Eligard.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocides
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Eligard
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after EXP.
The expiry date refers to the last day of the month stated.
Storage instructions
Store in a refrigerator (2°C to 8°C).
Store in the original packaging to protect from moisture.
Before administration, the medicinal product must be at room temperature. Remove it from the refrigerator about 30 minutes before preparation. After removing the medicinal product from the refrigerator, it can be stored in the original packaging at room temperature (below 25°C) for up to 4 weeks.
After the first opening of the plastic tray sealed with foil, the solution must be prepared immediately and administered to the patient as soon as possible. The product is for single use only.
Instructions for disposal of unused or expired Eligard
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Eligard contains
The active substance of Eligard is leuprolide acetate.
One ampoule-syringe (Syringe B) contains 45 mg of leuprolide acetate.
The other ingredients of the medicine are poly(DL-lactic-co-glycolic acid) (85:15) and N-methyl-2-pyrrolidone contained in the ampoule-syringe with solvent for solution for injection (Syringe A).
What Eligard looks like and contents of the pack
Eligard is a powder and solvent for solution for injection.
Eligard 45 mg is available in the following packs:
- A set consisting of a thermoformed tray sealed with foil and a sterile needle with a diameter of 18G, in a cardboard box. The tray contains a bag with a desiccant and a system of connected syringes consisting of: ampoule-syringe A containing solvent, ampoule-syringe B containing powder, and a connector with a latch for syringes A and B.
- A collective pack containing 2 sets, each containing one system of connected syringes.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Recordati Industria Chimica e Farmaceutica S.p.A.
Via Matteo Civitali 1
20148 Milan
Italy
For more detailed information, please contact your local representative of the marketing authorization holder:
Recordati Polska sp. z o.o.
Al. Armii Ludowej 26
00-609 Warsaw
phone: +48 22 206 84 50
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria:
Eligard Depot 45 mg
Belgium:
Depo-Eligard 45 mg
Bulgaria:
Eligard 45 mg
Cyprus:
Eligard
Czech Republic:
Eligard
Denmark:
Eligard
Estonia:
Eligard
Finland:
Eligard
France:
Eligard 45 mg
Germany:
Eligard 45 mg
Hungary:
Eligard 45 mg
Iceland:
Eligard
Ireland:
Eligard 45 mg
Italy:
Eligard
Latvia:
Eligard 45 mg
Lithuania:
Eligard 45 mg
Luxembourg:
Depo-Eligard 45 mg
Netherlands:
Eligard 45 mg
Norway:
Eligard
Poland:
Eligard 45 mg
Portugal:
Eligard 45 mg
Romania:
Eligard 45 mg
Slovakia:
Eligard 45 mg
Slovenia:
Eligard 45 mg
Spain:
Eligard Semestral 45 mg
Sweden:
Eligard
Date of last revision of the leaflet: 10/2024
Information intended for healthcare professionals only:
Before opening, the medicinal product should be brought to room temperature by removing it from the refrigerator about 30 minutes before use.
First, prepare the patient for administration, and then prepare the solution according to the instructions below. If the solution is not prepared using the appropriate technique, it should not be administered to the patient, as this may result in lack of clinical efficacy due to improper reconstitution of the medicinal product.
Step 1
On a clean surface, open the tray by tearing the foil starting from the corners to remove the contents. Remove the bag with the desiccant. Remove the system of connected syringes (Fig. 1.1) from the tray. Open the package containing the needle with a protective cover (Fig. 1.2) by tearing the paper part of the package.
Note: Syringe A and Syringe B should not be aligned yet.
Fig. 1.1
Tray contents: system of connected syringes
Fig. 1.2
Under the tray: Needle with protective cover and nozzle
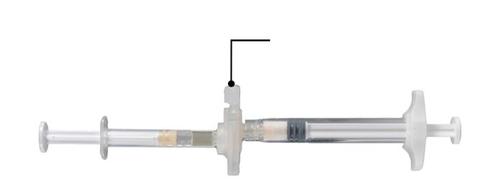

Step 2
Hold the latch on the connector with your thumb and index finger and press (Fig. 2) until you hear a click. The two syringes will be aligned. Activating the latch does not require any special position of the system of connected syringes. Do not bend the system of connected syringes (note that this may cause leakage, as the syringes may become partially unscrewed).
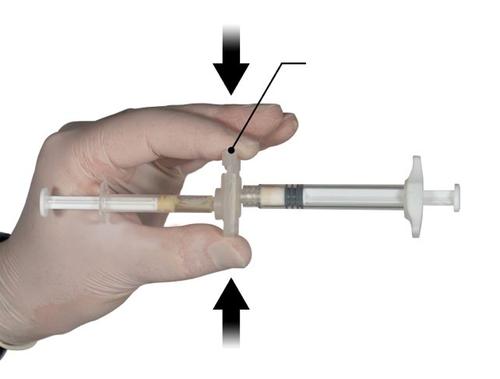
Step 3
Holding the syringes horizontally, move the liquid contents of Syringe A to the leuprolide acetate powder in Syringe B. Mix the product thoroughly by 60 cycles, gently moving the contents of both syringes between the two syringes (one cycle means one push of the Syringe A plunger and one push of the Syringe B plunger) with the syringes aligned horizontally, until a homogeneous, viscous solution is obtained (Fig. 3). Do not bend the system of connected syringes (note that this may cause leakage, as the syringes may become partially unscrewed).
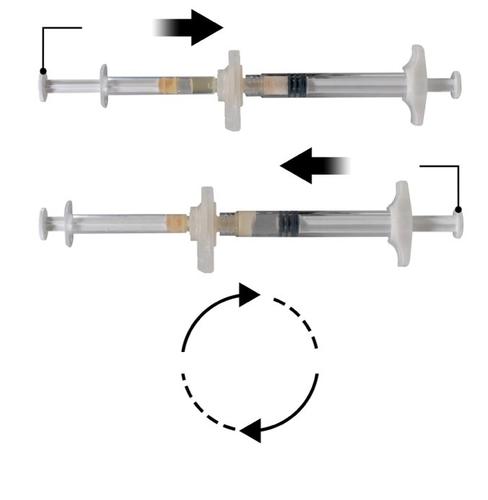
Repeat 60x
After thorough mixing, the viscous solution has a colorless to white or light yellow color (shades of white to light yellow).
Important: After mixing, proceed immediately to the next step, as the product's viscosity increases over time. Do not freeze the mixed product.
Note: The product must be mixed according to the instructions; shaking will NOT ensure proper mixing of the product.
Step 4:After mixing, hold the syringes vertically with Syringe B at the bottom. The syringes should remain properly connected. Move the entire mixed product to Syringe B (the large syringe) by pushing the Syringe A plunger and slightly pulling the Syringe B plunger (Fig. 4).
Fig. 4
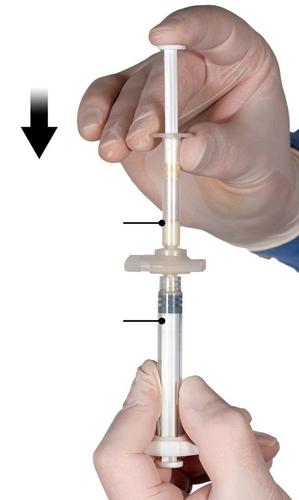
Step 5
Ensuring that the Syringe A plunger is fully depressed, hold the connector and unscrew it from Syringe B. Syringe A will remain connected to the connector (Fig. 5). Ensure that the product does not leak, as the needle, once attached, will not properly secure the syringe.
Note: One large or several small air bubbles may remain in the mixture - this is a normal phenomenon.
Do not remove air bubbles from Syringe B at this stage, as this may cause product loss!
Fig. 5
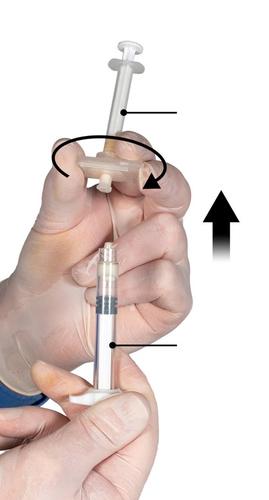
Step 6
- Hold Syringe B vertically and hold the white plunger to prevent product loss.
- Attach the needle with the protective cover to Syringe B, holding the syringe, and gently screw the needle about three-quarters of a turn in the direction of the arrow (Fig. 6). Do not overtighten, as this may cause the needle hub to break and result in product leakage during injection. If the needle is tightened too much, the protective cover may also be damaged.
If the needle hub is broken or appears damaged, or if leakage is observed, the medicinal product should not be administered. The damaged needle should not be replaced or exchanged, and the product should not be injected. All unused parts of the product should be disposed of safely.
In case of needle hub damage, a new medicinal product should be administered.
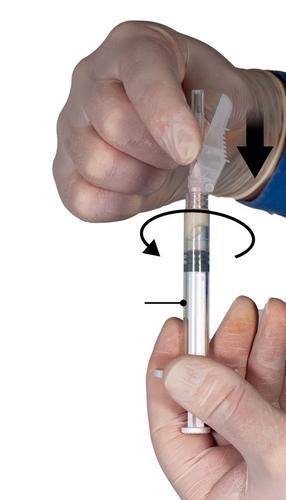
Step 7
Bend the protective cover away from the needle and remove the needle cover immediately before administering the medicinal product (Fig. 7).
Important: Before administration, do not manipulate the needle protective cover mechanism. If the needle hub appears damaged or leakage is observed, DO NOT use the product. The damaged needle should NOT be replaced, and the product should NOT be injected. In case of needle hub damage, a new Eligard medicinal product set should be used.
Fig. 7
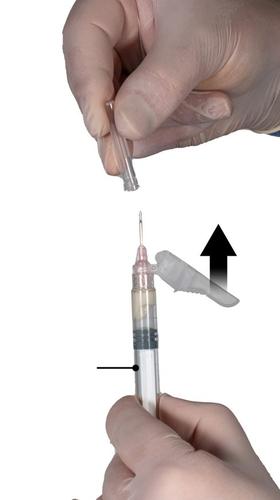
Step 8
Before administering the medicinal product, remove all largeair bubbles from Syringe B. Administer the product subcutaneously, keeping the protective cover away from the needle.
Administration procedure:
Fig. 8
- Choose an injection site that has not been used recently, on the abdomen, in the upper buttock area, or another location with sufficient subcutaneous tissue and without discoloration, nodules, changes, or hair.
- Clean the injection site area with an alcohol swab (not included).
- With your thumb and index finger, grasp and squeeze the skin around the injection site.
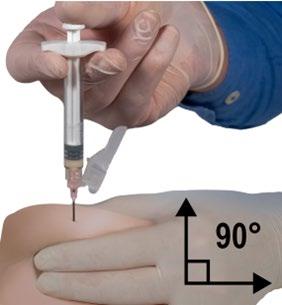
- With your dominant hand, quickly insert the needle at a 90° angle relative to the skin surface. The depth of penetration will depend on the amount and density of subcutaneous tissue and the length of the needle. After inserting the needle, release the skin.
- Inject the product slowly and evenly by pushing the plunger until the syringe is empty. Before removing the needle, ensure that the entire amount of product has been injected from Syringe B.
- Maintaining pressure on the plunger, quickly withdraw the needle at the same 90° angle used for insertion.
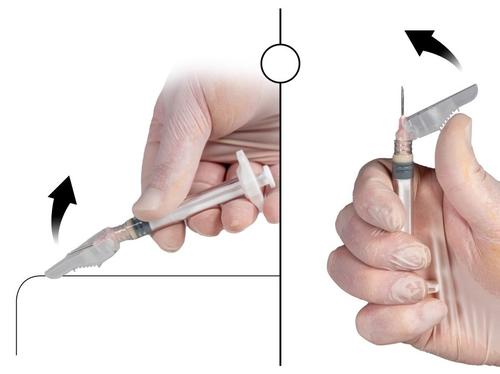
Step 9
After injecting the medicinal product, close the needle protective cover using one of the methods listed below.
1. Closing on a flat surface
Press the protective cover, with the lever facing down, against a flat surface (Fig. 9a) to cover the needle and close the protective cover.
The cover is closed if you hear and feel a click. In the closed position, the needle tip will be completely covered.
2. Closing with the thumb
Place your thumb on the protective cover (Fig. 9b), cover the needle tip, and close the protective cover.
The cover is closed if you hear and feel a click. In the closed position, the needle tip will be completely covered.
Fig. 9a
Closing on a flat surface
Fig. 9b
Closing with the thumb
After closing the protective cover, immediately discard the needle and syringe into a sharps container.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterRecordati Industria Chimica e Farmaceutica S.p.A
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Eligard 45 mgDosage form: Powder, 22.5 mgActive substance: leuprorelinPrescription requiredDosage form: Powder, 7.5 mgActive substance: leuprorelinPrescription requiredDosage form: Implant, 3.6 mgActive substance: leuprorelinPrescription required
Alternatives to Eligard 45 mg in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Eligard 45 mg in Spain
Alternative to Eligard 45 mg in Ukraine
Online doctors for Eligard 45 mg
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Eligard 45 mg – subject to medical assessment and local rules.











