LEUPRORELIN GP-PHARM DEPOT MONTHLY 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION

How to use LEUPRORELIN GP-PHARM DEPOT MONTHLY 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Leuprorelin GP-Pharm Depot Monthly and what is it used for
- What you need to know before you use Leuprorelin GP-Pharm Depot Monthly
- Do not use Leuprorelin GP-Pharm
- How to use Leuprorelin GP-Pharm Depot Monthly
- Dosage
- Possible side effects
- Storage of Leuprorelina GP-Pharm Depot Monthly
- Package contents and additional information
- Composition of Leuprorelina GP-Pharm
Introduction
Package Leaflet: Information for the User
Leuprorelin GP-Pharm Depot Monthly3.75 mg powder and solvent for prolonged-release suspension for injection
Leuprorelin acetate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Leuprorelin GP-Pharm Depot Monthly and what is it used for
- What you need to know before you use Leuprorelin GP-Pharm Depot Monthly
- How to use Leuprorelin GP-Pharm Depot Monthly
- Possible side effects
- Storage of Leuprorelin GP-Pharm Depot Monthly
- Contents of the pack and other information
1. What is Leuprorelin GP-Pharm Depot Monthly and what is it used for
Leuprorelin GP-Pharm is a vial containing a white powder, which is reconstituted as a suspension for injection into a muscle. Leuprorelin GP-Pharm contains the active substance leuprorelin (also known as leuprolide), which belongs to a group of medicines called gonadotropin-releasing hormone (LHRH) agonists, which are medicines that reduce testosterone (a sex hormone).
Your doctor has prescribed Leuprorelin GP-Pharm for:
- Palliative treatment of advanced prostate cancer in men.
- Treatment of endometriosis for a period of up to six months. It may be used alone or as combination therapy with surgery.
- Treatment of uterine fibroids for a period of up to 6 months. This treatment may be used as a preoperative measure or as adjuvant therapy to surgery or as an alternative definitive symptomatic treatment in perimenopausal women who do not desire surgery.
- Treatment of hormone-sensitive breast cancer in early stage in pre- and perimenopausal women with high risk of recurrence.
- Treatment of advanced hormone-sensitive breast cancer in pre- and perimenopausal women.
- Preservation of ovarian function in premenopausal women with cancer who are receiving chemotherapy.
- In children: treatment of central precocious puberty (in girls under 9 years, in boys under 10 years).
2. What you need to know before you use Leuprorelin GP-Pharm Depot Monthly
Do not use Leuprorelin GP-Pharm
- If you are allergic (hypersensitive) to LHRH, LHRH agonists or any of the other ingredients of this medicine (listed in section 6). An allergic reaction may appear as a skin rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue.
- In men with prostate cancer:
- If you have undergone orchiectomy (removal of the testicles).
- Leuprorelin GP-Pharm should not be used alone for the treatment of prostate cancer when the spinal cord is compressed or the cancer has spread to the spinal cord.
- In women:
- If you are pregnant, plan to become pregnant or are breastfeeding.
- If you have abnormal vaginal bleeding that you have not discussed with your doctor.
- In pre- and perimenopausal women receiving Leuprorelin GP-Pharm for breast cancer:
Your estrogen levels must have been adequately suppressed with Leuprorelin GP-Pharm before starting treatment with an aromatase inhibitor such as exemestane and must be monitored every three months during combined treatment with Leuprorelin GP-Pharm and an aromatase inhibitor (see the section "Warnings and precautions" below for more information).
- In girls with central precocious puberty:
- if the girl to be treated is pregnant or breastfeeding.
- if the girl suffers from undiagnosed vaginal bleeding.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Leuprorelin GP-Pharm.
Tell your doctor if you suffer from any of the following symptoms:
Men and women
- If you think you are experiencing an allergic reaction (shortness of breath, asthma, rhinitis, swelling of the face, urticaria, skin rash), stop taking this medicine and inform your doctor.
- Depression has been reported in patients treated with Leuprorelin GP-Pharm, which may be severe. If you are using Leuprorelin GP-Pharm and feel depressed, inform your doctor.
- Tell your doctor if you are at risk of or already suffer from any of the following diseases, as you may need more frequent check-ups:
- Bruises or unexplained bleeding or if you feel unwell. Although rare, these can be symptoms of changes in the number of red or white blood cells.
- Metabolic disease
- Heart problems, or palpitations
- Diabetes
- Your doctor should be informed of any personal clinical history of pituitary adenoma (non-malignant tumor of the pituitary gland). Cases of pituitary apoplexy (partial loss of pituitary tissue) have been described after administration of this type of medicine to patients with pituitary adenoma. Pituitary apoplexy may manifest as sudden headache, meningism, vision disorders or altered vision, including blindness, and occasionally a decrease in the level of consciousness.
- It is possible that your liver function may need to be monitored, as liver disorders and jaundice (yellowing of the eyes and skin) have been described with the administration of leuprorelin.
- Seizures may occur in predisposed patients (patients with a history of seizures, epilepsy, cerebrovascular disorders, anomalies or tumors of the central nervous system), in patients taking medications that may cause seizures, and to a lesser extent in patients who do not have these characteristics.
- Leuprorelin GP-Pharm contains an ingredient that could give a positive result in doping tests.
- Contact your doctor immediately if you (or your child) suffer from intense or recurrent headaches, vision problems, and tinnitus or ringing in the ears.
- Severe skin reactions, including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), have been reported in association with leuprorelin. Discontinue use of leuprorelin and seek immediate medical attention if you notice any of the symptoms related to these severe skin reactions described in section 4.
Only men
- Tell your doctor if you have any heart or blood vessel disorders or are being treated for them, including medications to control heart rhythm (arrhythmias). The risk of heart rhythm problems may increase when using leuprorelin acetate.
- Your disease may worsen during the first few weeks of treatment, but it should improve with continued treatment. The signs and symptoms include: temporary increase in testosterone (male hormone), hot flashes, bone pain, nervous system disorders (including depression) or urinary obstruction.
- Your doctor should know if you suffer from a coagulation disorder, thrombocytopenia, or if you are being treated with anticoagulants.
- Fracture of the spine, paralysis, low blood pressure, and high blood pressure have been described with leuprorelin treatment.
- A decrease in bone density (fragile or less dense bones) has been described after administration of leuprorelin. Your doctor may consider adding an anti-androgen to the treatment with Leuprorelin GP-Pharm. In this case, the doctor will be alert to detect the presence of vein inflammation (thrombophlebitis) and other signs of coagulation disorders and edema (swelling of hands, feet, or ankles) that are more likely to occur when anti-androgenic treatment is added to Leuprorelin GP-Pharm.
- Tell your doctor if you feel pressure on the spinal cord and/or present urinary disorders and/or hematuria (blood in the urine); in such a case, the doctor will discuss with you the need for additional treatments to prevent neurological complications (e.g., tingling in hands and feet, paralysis) or urethral obstruction (the duct that connects the bladder to the outside of the body). You will be closely monitored during the first few weeks of treatment.
- Patient may experience metabolic changes (e.g., glucose intolerance or worsening of existing diabetes), weight changes, and cardiovascular disorders.
- Patient with metabolic or cardiovascular disease, and especially those with a history of congestive heart failure (a disease in which the heart is no longer able to pump enough blood to the rest of the body), should be monitored during treatment with leuprorelin.
- Consult your doctor, pharmacist, or nurse if you have fatty liver.
- During treatment, some blood tests will be performed to check if Leuprorelin GP-Pharm is effective.
- You may experience a loss of interest in sexual relations, hot flashes, and occasionally a reduction in the size and function of the testicles may occur.
- You may become fertile again when treatment with Leuprorelin GP-Pharm is discontinued.
- Leuprorelin GP-Pharm may interfere with certain laboratory tests, so you should ensure that your doctor knows that you are using Leuprorelin GP-Pharm.
Only women
- A decrease in bone density (fragile or less dense bones) has been reported with leuprorelin, which is reversible after completing a six-month cycle of leuprorelin acetate. If you have a higher risk of developing less dense bones (osteoporosis), you should inform your doctor before taking Leuprorelin GP-Pharm. Risk factors include:
- If you or a close family member has osteoporosis.
- If you drink excessive amounts of alcohol and/or smoke heavily.
- If you take medications for a prolonged period that may cause less dense bones, such as medications for epilepsy or steroids (such as hydrocortisone or prednisolone).
- Your condition may worsen at the beginning during the first few weeks of treatment, but it should improve with continued treatment.
- If you are a woman with submucous fibroids (benign tumors in the muscle under the lining of the uterus), Leuprorelin GP-Pharm may cause severe bleeding when the fibroids break down. Contact your doctor immediately if you suffer from severe or unusual bleeding or pain.
- If you are a woman of childbearing age, you should use a non-hormonal contraceptive method while receiving Leuprorelin GP-Pharm. Although Leuprorelin GP-Pharm interrupts menstruation, it is not a contraceptive in itself. If you are unsure about this, talk to your doctor.
- If you are a woman and continue to have periods (menstruation) after starting treatment with Leuprorelin GP-Pharm, you should inform your doctor.
- If you are receiving this medication for the treatment of breast cancer:
- Your doctor may evaluate your bone density and ovarian function before starting treatment with Leuprorelin GP-Pharm and monitor your bone density and ovarian function during treatment.
- Leuprorelin GP-Pharm should be started at least 6-8 weeks before starting treatment with an aromatase inhibitor and should continue throughout treatment with the aromatase inhibitor.
- If you have received chemotherapy, treatment with Leuprorelin GP-Pharm should only begin once chemotherapy has been completed and premenopausal status has been confirmed.
- The recommended duration of treatment with Leuprorelin GP-Pharm in combination with other hormonal treatments for breast cancer is up to 5 years.
- If you are administered Leuprorelin GP-Pharm in combination with an aromatase inhibitor, your doctor may monitor your blood pressure, heart function, and blood glucose levels during treatment. If you have depression or a history of depression, inform your doctor so that they can also monitor your symptoms of depression during treatment with Leuprorelin GP-Pharm.
- If you are unsure about any of these points, talk to your doctor.
In children:
- In case of a sterile abscess at the injection site, your doctor will monitor your hormone levels as there may be a reduction in the absorption of leuprorelin at the injection site.
- If the child has a progressive brain tumor, the doctor will decide whether treatment with leuprorelin is appropriate.
- Severe skin reactions, including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), have been reported in association with leuprorelin. Discontinue use of leuprorelin and seek immediate medical attention if you notice any of the symptoms related to these severe skin reactions described in section 4.
In girls with central precocious puberty
- After the first injection, vaginal bleeding (spotting) and vaginal discharge may appear as a sign of hormonal withdrawal. Vaginal bleeding beyond the first/second month of treatment must be investigated.
- During treatment for central precocious puberty with Leuprorelin GP-Pharm, bone density may decrease. Despite this, after treatment is completed, subsequent bone mass accumulation is maintained and the peak bone mass in late adolescence does not appear to be altered by treatment.
- After treatment is discontinued, slipping of the femoral epiphysis may occur. A possible cause of the weakness of the growth plate is the decrease in female sex hormone concentrations during treatment.
Using Leuprorelin GP-Pharm with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. It may still be suitable to use Leuprorelin GP-Pharm; your doctor will decide what is suitable for you.
Leuprorelin GP-Pharm may interfere with certain medications used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with other medications (e.g., methadone (used for pain relief and for detoxification from other medications), moxifloxacin (an antibiotic), antipsychotics used to treat serious mental illnesses).
Pregnancy and breastfeeding
Leuprorelin GP-Pharm is contraindicated during pregnancy. Spontaneous abortions may occur if this medicine is administered during pregnancy.
It is not known whether leuprorelin acetate is excreted in breast milk, therefore, Leuprorelin GP-Pharm should not be administered to women or girls who are breastfeeding.
Driving and using machines
Visual disturbances and dizziness may occur during treatment. If you are affected, do not drive or operate machinery.
Leuprorelin GP-Pharm containsless than 23 mg of sodium (1 mmol) per dose; this is, essentially “sodium-free”.
3. How to use Leuprorelin GP-Pharm Depot Monthly
Dosage
Leuprorelina GP-Pharm should only be administered by your doctor or nurse. They will be responsible for preparing the product.
Adults and elderly:
The recommended dose of Leuprorelina GP-Pharm is one injection once a month. The powder is reconstituted to form a suspension that is administered as an intramuscular injection (in a muscle) once a month (approximately every 28-33 days).
The injection site should be varied at regular intervals.
Leuprorelina GP-Pharm should only be administered intramuscularly. It should not be administered by any other route.
The treatment regimen will be decided by your doctor.
If you have endometriosis or uterine fibroids, you will be given an injection of Leuprorelina GP-Pharm for a period of up to 6 months at most.
If you have breast cancer, you will be given Leuprorelina GP-Pharm once a month in combination with tamoxifen or an aromatase inhibitor. Before starting treatment with an aromatase inhibitor or tamoxifen, you must have received at least two injections of Leuprorelina GP-Pharm with a one-month interval between each injection.
If you are given Leuprorelina GP-Pharm to preserve ovarian function while receiving chemotherapy, you will normally be given an injection of Leuprorelina GP-Pharm two weeks before starting chemotherapy and then every month during the duration of your chemotherapy treatment.
Use in children:
Treatment of children should be under the general supervision of a pediatric endocrinologist.
Dose adjustment should be done individually.
The recommended starting dose depends on body weight:
- Children with a body weight of 20 kg or more
Unless your doctor tells you otherwise, 2 ml of Leuprorelina GP-Pharm (3.75 mg of leuprorelin acetate) will be administered once a month as a single intramuscular injection.
- Children with a body weight of less than 20 kg
Taking into account the clinical activity of central precocious puberty in these rare cases, the following procedure should be followed:
Unless your doctor tells you otherwise, 1 ml of Leuprorelina GP-Pharm (1.88 mg of leuprorelin acetate) will be administered once a month as a single intramuscular injection. The rest of the suspension should be discarded. Your doctor will monitor the child's weight gain.
Depending on the progression of central precocious puberty, your doctor may increase the dose if inadequate suppression is observed (e.g., vaginal bleeding). Your doctor will determine the minimum effective dose through a blood test.
The duration of treatment depends on the clinical symptoms at the start of treatment or during treatment and is decided jointly between the treating doctor, the legal guardian, and, if appropriate, the treated child. Your doctor will determine the child's bone age at regular intervals.
In girls with bone maturation of more than 12 years and boys with bone maturation of more than 13 years, your doctor will assess stopping treatment based on the clinical effects on your child.
In girls, pregnancy should be ruled out before starting treatment. Generally, pregnancy cannot be excluded during treatment. In these cases, consult your doctor.
Treatment is long-term and is adjusted individually. Please agree with your doctor that the administration of Leuprorelina GP-Pharm be done as precisely as possible at regular monthly intervals. An exceptional delay of the injection date by a few days (30 ± 2 days) does not affect the outcome of the treatment.
If you use more Leuprorelina GP-Pharm than you should
This is unlikely, as the doctor or nurse will know what the correct dose is. Nevertheless, if you suspect that you have received more medication than you should, inform your doctor immediately so that the necessary measures can be taken.
In case of overdose or accidental ingestion, consult the Toxicological Information Service, tel: 91 562 04 20.
If you miss a dose of Leuprorelina GP-Pharm
It is essential that you do not miss a dose of Leuprorelina GP-Pharm. As soon as you realize that you have missed an injection, contact your doctor, who will administer the next injection.
Only for women:if you miss an injection of Leuprorelina GP-Pharm, intermenstrual bleeding or ovulation with the possibility of conception may occur. If you think you may be pregnant, you should stop using Leuprorelina GP-Pharm and contact your doctor immediately.
If you interrupt treatment with Leuprorelina GP-Pharm
Since medical treatment involves the administration of Leuprorelina GP-Pharm for a long period, interrupting treatment may result in a worsening of symptoms related to the disease. Therefore, you should not interrupt treatment prematurely without your doctor's permission.
If you are given Leuprorelina GP-Pharm for the treatment of breast cancer, you should not interrupt treatment with Leuprorelina GP-Pharm while taking an aromatase inhibitor or tamoxifen. If you are going to interrupt treatment with Leuprorelina GP-Pharm, your treatment with the aromatase inhibitor should also be interrupted within 1 month from the last injection of Leuprorelina GP-Pharm.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Leuprorelina GP-Pharm can cause side effects, although not everyone gets them.
Tell your doctor immediately if you notice any of the following symptoms:
- You experience wheezing, difficulty breathing, swelling of the eyelids, face, or lips, skin rash, or itching (especially if it affects the whole body) suddenly.
- Frequency not known (frequency cannot be estimated from available data):
- You notice circular or target-shaped spots on the trunk, reddish in color, and not elevated, often with central blisters, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin rashes can be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome/toxic epidermal necrolysis).
- Redness of the skin and rash with itching (toxic skin rash).
- A skin reaction that causes grains or spots on the skin, which can look like a target, with a dark red center surrounded by lighter red rings (erythema multiforme).
During the first few weeks of treatment, a transient worsening of your condition may occur, but it should improve with continued treatment.
Men
The following side effects have been described:
Very common (may affect more than 1 in 10 people):
Hot flashes and reactions at the injection site.
Common (may affect up to 1 in 10 people):
Nocturnal sweats, cold sweats, fatigue, headache, pyrexia (increased body temperature), increased appetite, erectile dysfunction, hyperhidrosis (increased sweating), asthenia (lack or loss of strength), back pain, and reactions at the injection site such as pain, irritation, discomfort, erythema (redness of the skin), swelling (increase in size or inflammation), hematoma (bruising), and mood changes and depression in prolonged treatments with leuprorelin.
Uncommon (may affect up to 1 in 100 people):
Breast swelling, breast pain, dizziness, weakness, sleep disorders, somnolence, insomnia, lower abdominal pain, diarrhea, nausea, vomiting, sensation of cold and heat, restlessness, fever, yellowing of the eyes and skin (jaundice), alteration of liver enzymes, anorexia, high cholesterol, joint pain, muscle spasms, pain in hands and feet, decreased sexual desire, mood changes, urinary retention, frequent urination, urinary incontinence, swelling around the eyes, ejaculation disorder, hyperlipidemia (high levels of lipids in the blood), pruritus (itching), urticaria (skin rash), mood changes, and depression in short-term treatments with leuprorelin, reactions at the injection site such as swelling, wound, and bleeding.
Not known (frequency cannot be estimated from available data):
Changes in the ECG (prolongation of the QT interval)
Inflammation of the lungs, lung disease
Idiopathic intracranial hypertension (increased pressure around the brain characterized by headaches, double vision, and other visual symptoms, ringing or buzzing in one or both ears).
Women
Many of the side effects of Leuprorelina GP-Pharm are related to the decrease in estrogen levels. Estrogen levels return to normal after stopping treatment. Common side effects that may occur include hot flashes, mood changes, depression, and vaginal dryness. As can happen naturally when women reach menopause, Leuprorelina GP-Pharm may cause a small reduction in bone thickness. Vaginal bleeding may occur during treatment.
The following side effects have been described:
Very common (may affect more than 1 in 10 people):
Difficulty sleeping, headache, or hot flashes.
Common (may affect up to 1 in 10 people):
Weight changes, mood changes, depression, tingling in hands or feet, dizziness, nausea, joint pain, muscle weakness, breast tenderness, changes in breast size, vaginal dryness, swelling of the ankles, or skin reactions at the injection site (including skin hardening, redness, pain, abscesses, swelling, nodules, ulcers, and skin damage).
Uncommon (may affect up to 1 in 100 people):
Lack of appetite, changes in blood lipids (cholesterol), vision changes, strong heartbeats, diarrhea, vomiting, abnormal liver values in blood tests, hair loss, muscle pain, fever, chills, or fatigue.
Not known (frequency cannot be estimated from available data):
Blood tests may show anemia (low red blood cell count), low white blood cell count, or low platelet count, allergic reactions (including symptoms of rash, itching, hives, or a severe allergic reaction that causes difficulty breathing or dizziness), changes in blood sugar levels, paralysis, blood clots in the lungs, high or low blood pressure, jaundice (yellowing of the skin), liver function abnormalities, spinal fracture, seizures, less dense bones, or vaginal bleeding, lung inflammation, or lung disease.
Idiopathic intracranial hypertension (increased pressure around the brain characterized by headaches, double vision, and other visual symptoms, ringing or buzzing in one or both ears).
Side effects when used for the treatment of breast cancer in combination with tamoxifen or an aromatase inhibitor
The following side effects have been observed when a similar class of medications called GnRH analogs (gonadotropin-releasing hormone analogs) has been used for breast cancer in combination with tamoxifen or an aromatase inhibitor:
Very common (may affect more than 1 in 10 people):
Nausea, feeling very tired, joint and muscle pain, osteoporosis, hot flashes, excessive sweating, difficulty sleeping, depression, decreased libido, vaginal dryness, pain during or after intercourse, urinary incontinence, increased blood pressure.
Common (may affect up to 1 in 10 people):
Diabetes, high blood sugar levels (hyperglycemia), pain, bruising, redness, and swelling at the injection site, allergic reaction, bone fractures, blood clots in a blood vessel.
Uncommon (may affect up to 1 in 100 people):
Bleeding in the brain, lack of blood flow to the brain or heart.
Rare (may affect up to 1 in 1000 people):
Changes in the ECG (prolongation of the QT interval).
Children
In the initial phase of treatment, there is a short-term increase in sex hormone levels, followed by a drop in values within the prepubertal range. Due to this, side effects may occur especially at the start of treatment.
The following side effects have been described:
Common (may affect up to 1 in 10 patients):
Mood changes, headache, abdominal pain/cramps, nausea/vomiting, acne, vaginal bleeding, spotting, vaginal discharge, reactions at the injection site.
Very rare (may affect up to 1 in 10,000 people):
Generalized allergic reactions (fever, skin rash, itching), severe allergic reactions that cause difficulty breathing or dizziness.
As with other medications in this class: if you have an existing pituitary lesion, there may be a higher risk of bleeding in the area, which could cause permanent damage.
Not known (frequency cannot be estimated from available data):
Seizures, lung inflammation, lung disease.
Idiopathic intracranial hypertension (increased pressure around the brain characterized by headaches, double vision, and other visual symptoms, ringing or buzzing in one or both ears).
Notes:
Generally, the occurrence of vaginal bleeding during continued treatment (after the possible withdrawal bleeding in the first month of treatment) should be evaluated as a potential sign of underdosing. If vaginal bleeding occurs, inform your doctor.
Reporting side effects:
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Leuprorelina GP-Pharm Depot Monthly
Your doctor or pharmacist will know how to store Leuprorelina GP-Pharm.
Keep this medication out of sight and reach of children.
Do not store above 25°C. Do not freeze.
Store in the original packaging to protect from light.
Do not use this medication after the expiration date shown on the packaging, vial, and syringe after "EXP". The syringe has the same expiration date as the vial.
The expiration date is the last day of the month indicated.
Once reconstituted with the solvent, the suspension should be administered immediately.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and unused medicines in the SIGRE collection point at the pharmacy. Ask your pharmacist how to dispose of the packaging and unused medicines. This will help protect the environment.
6. Package contents and additional information
Composition of Leuprorelina GP-Pharm
The active ingredient is leuprorelina acetate. Each vial contains 3.75 mg of leuprorelina acetate.
The other components are: polysorbate 80, mannitol (E-421), sodium carmellose (E-466), triethyl citrate, and poly(DL-lactic-co-glycolic acid) (PLGA).
The solvent contains (pre-filled syringe): mannitol, water for injectables, sodium hydroxide (for pH adjustment), and hydrochloric acid (for pH adjustment).
The concentration of the reconstituted product is 1.875 mg/ml.
Appearance of the product and package contents
Each package contains a vial with 3.75 mg of leuprorelina acetate, a pre-filled syringe with 2 ml of solvent, an adapter system, and a sterile 20-gauge needle.
Marketing authorization holder and manufacturer
GP-PHARM, S.A.
Pol.Ind. Els Vinyets – Els Fogars Sector 2
Carretera comarcal 244, km22
08777 Sant Quintí de Mediona
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Spain: Leuprorelina GP-Pharm Depot Monthly 3.75 mg powder and solvent for prolonged-release injectable suspension
Portugal: Lutrate Depot 3.75 mg / 2 ml powder and vehicle for injectable suspension of prolonged release
Greece: Lutrate Depot 3.75 mg Κ?νις και διαλ?της για παρασκευ? ενεσ?μου εναιωρ?ματος παρατεταμ?νης αποδ?σμευσης
Italy: Politrate
Hungary: Politrate Depot 3.75 mg
Date of the last revision of this leaflet: June 2024
Detailed and updated information on this medicinal productis available on the website of the Spanish Agency for Medicines and Health Products (AEMPS)http://www.aemps.gob.es/.
This information is intended solely for healthcare professionals.
How to prepare the injection?
IMPORTANT: Read carefully before administering the product (the Instructions for use are also included in the tray containing the components of the kit).
Aseptic technique should be followed during the reconstitution procedure.
Use only the solvent included in the commercial kit.
Once mixed, the product must be administered immediately by single intramuscular injection.
This medicinal product is for single use. Any remaining suspension should be discarded.
Check the contents of the kit and ensure that it includes everything mentioned in the leaflet.
The package contains:
1 (one) vial of Leuprorelina GP-Pharm Depot Monthly 3.75 mg (leuprorelina acetate) powder for injectable suspension
1 (one) pre-filled syringe containing the solvent for the suspension (mannitol 0.8% injectable solution)
1 (one) sterile single-use reconstitution device, including 1 (one) sterile needle.
1 |
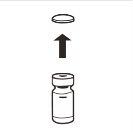
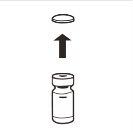
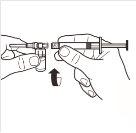
| Completely remove the pressure cap from the top of the vial, so that the rubber stopper is exposed. Confirm that no parts of the pressure cap remain in the vial. |
2 |
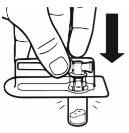
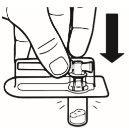
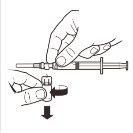
| Place the vial in a vertical position on a table. Remove the blister cover containing the vial adapter (MIXJECT). Do not remove the vial adapter from the blister.Firmly place the blister containing the vial adapter on the top of the vial, perforating the stopperin a fully vertical position. Press gently downwardsuntil it clicks into place. |
3 |
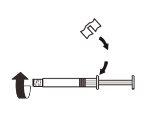
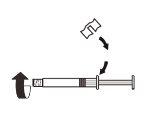
| Fix the white piece to the syringe until it clicks. Unscrewthe rigid cap of the syringe in an anti-clockwise direction. Then, remove the blister from the MIXJECT adapter system. |
4 |
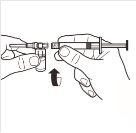
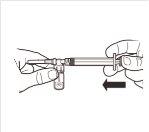
| Connect the syringe to the adapter system by screwing it in a clockwise direction into the lateral opening of the adapter system. To ensure a hermetic connection, screw the syringe gently until it stops. |
5 |
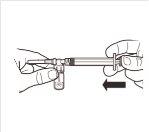
| While keeping the syringe and vial firmly together in a vertical position, slowly push the syringe plunger to transfer all the solvent to the vial. |
6 |
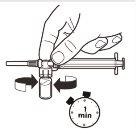
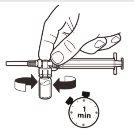
| With the syringe still attached to the vial, gently shake the vial for approximately 1 minuteuntil a uniform milky suspension is obtained.To avoid separation of the suspension, perform the following steps without stopping. |
7 |
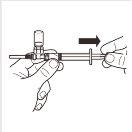
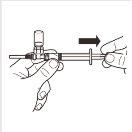
| Turn the MIXJECT adapter system so that the vial is at the top. Hold the MIXJECT adapter system firmly by the syringe and slowly pull the plunger to transfer the contents of the vial to the syringe. Part of the product may accumulate or be deposited on the wall of the vial. This is normal. |
8 |
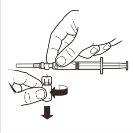
| Disconnect the syringe from the MIXJECT adapter system. To do this, hold the syringe firmly and turn the vial in a clockwise direction (holding the plastic cap of the adapter system). |
9 |
| Keep the syringe IN A VERTICAL POSITION. With the other hand, remove the needle protector by pulling upwards. Press the plunger slightly to expel the air from the syringe. The syringe containingthe product is ready for immediate administration. |
10 |
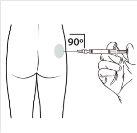
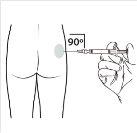
| Administer the intramuscular injection by inserting the needle at a 90-degree angle into the buttock. Ensurethat the entire product is injected.The injection sites should be alternated. |
Instructions for use
To be included in the cover of the tray containing the components of the Medicinal Product Kit
Leuprorelina GP-Pharm Depot–Instructions for use
Read carefully before administering the product
Reconstitute immediately before administering by single intramuscular injection
Use only the solvent included in the commercial kit.
Product intended for single injection.
Any remaining suspension should be discarded.
1 |
| Completely remove the pressure cap from the top of the vial, so that the rubber stopper is exposed. Confirm that no parts of the pressure cap remain in the vial. |
2 |
| Place the vial in a vertical position on a table. Remove the blister cover containing the vial adapter (MIXJECT). Do not remove the vial adapter from the blister.Firmly place the blister containing the vial adapter on the top of the vial, perforating the stopperin a fully vertical position. Press gently downwardsuntil it clicks into place. |
3 |
| Fix the white piece to the syringe until it clicks. Unscrewthe rigid cap of the syringe in an anti-clockwise direction. Then, remove the blister from the MIXJECT adapter system. |
4 |
| Connect the syringe to the adapter system by screwing it in a clockwise direction into the lateral opening of the adapter system. To ensure a hermetic connection, screw the syringe gently until it stops. |
5 |
| While keeping the syringe and vial firmly together in a vertical position, slowly push the syringe plunger to transfer all the solvent to the vial. |
6 |
| With the syringe still attached to the vial, gently shake the vial for approximately 1 minuteuntil a uniform milky suspension is obtained.To avoid separation of the suspension, perform the following steps without stopping. |
7 |
| Turn the MIXJECT adapter system so that the vial is at the top. Hold the MIXJECT adapter system firmly by the syringe and slowly pull the plunger to transfer the contents of the vial to the syringe. Part of the product may accumulate or be deposited on the wall of the vial. This is normal. |
8 |
| Disconnect the syringe from the MIXJECT adapter system. To do this, hold the syringe firmly and turn the vial in a clockwise direction (holding the plastic cap of the adapter system). |
9 |
| Keep the syringe IN A VERTICAL POSITION. With the other hand, remove the needle protector by pulling upwards. Press the plunger slightly to expel the air from the syringe. The syringe containing the productis ready for immediate administration. |
10 |
| Administer the intramuscular injection by inserting the needle at a 90-degree angle into the buttock. Ensurethat the entire product is injected.The injection sites should be alternated. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEUPRORELIN GP-PHARM DEPOT MONTHLY 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSIONDosage form: INJECTABLE, 42 mgActive substance: leuprorelinManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 45 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription requiredDosage form: INJECTABLE, 22.5 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription required
Online doctors for LEUPRORELIN GP-PHARM DEPOT MONTHLY 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION
Discuss questions about LEUPRORELIN GP-PHARM DEPOT MONTHLY 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions