
ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ

Запитайте лікаря про рецепт на ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ

Інструкція із застосування ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ
Вступ
Опис:інформація для пацієнта
Таноналла 5 мг/2,5 мг пролонговані таблетки EFG
гідрохлорид оксикодону/гідрохлорид налоксону
Прочитайте уважно весь опис перед початком прийому цього лікарського засобу,адже він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас виникли питання, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей лікарський засіб призначений тільки для вас, і не передавайте його іншим людям, навіть якщо вони мають такі самі симптоми, як у вас, оскільки це може їм зашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке Таноналла і для чого вона використовується
- Що потрібно знати перед початком прийому Таноналли
- Як приймати Таноналлу
- Можливі побічні ефекти
- Зберігання Таноналли
- Зміст упаковки та додаткова інформація
1. Що таке Таноналла і для чого вона використовується
Таноналла - це пролонговані таблетки, що означає, що їх активні речовини вивільняються протягом тривалого періоду часу. Її дія триває 12 годин.
Ці таблетки призначені тільки для дорослих.
Зняття болю
Вам призначили Таноналлу для лікування сильного болю, який можна адекватно лікувати тільки опіоїдними анальгетиками. До складу препарату входить гідрохлорид налоксону для боротьби зі запорами.
Як ці таблетки працюють для зняття болю
Таноналла містить активні речовини гідрохлорид оксикодону та гідрохлорид налоксону. Гідрохлорид оксикодону відповідає за анальгетичну дію Таноналли, і це потужний анальгетик групи опіоїдів.
Друга активна речовина Таноналли, гідрохлорид налоксону, має функцію боротьби з запорами. Дисфункція кишечника (наприклад, запор) - це частий побічний ефект лікування опіоїдними анальгетиками.
2. Що потрібно знати перед початком прийому Таноналли
Не приймайте Таноналлу:
- якщо ви алергічні на гідрохлорид оксикодону, гідрохлорид налоксону або на будь-які інші компоненти цього лікарського засобу (перелічені в розділі 6),
- якщо у вас є проблеми з диханням, такі як повільне або слабке дихання (депресія дихання),
- якщо ви страждаєте на важку хворобу легенів, пов'язану з звуженням дихальних шляхів (хронічна обструктивна хвороба легенів або ХОХЛ),
- якщо ви страждаєте на захворювання, яке називається кор пульмонале. Це захворювання полягає в тому, що правий бок серця збільшується в розмірі через підвищення тиску в легенях, тощо. (наприклад, внаслідок ХОХЛ, див. вище),
- якщо ви страждаєте на важкий бронхіальний астму,
- якщо ви страждаєте на паралітичний ілеус (тип кишкової непрохідності) не викликаний опіоїдами,
- якщо ви страждаєте на захворювання печінки помірної чи важкої тяжкості.
Попередження та застереження
Проконсультуйтеся з вашим лікарем або фармацевтом перед початком прийому Таноналли:
- якщо ви пацієнт похилого віку або ослаблений (слабкий),
- якщо ви страждаєте на паралітичний ілеус (тип кишкової непрохідності) викликаний опіоїдами,
- якщо ви страждаєте на захворювання нирок,
- якщо ви страждаєте на легке захворювання печінки,
- якщо ви страждаєте на важке захворювання легенів (наприклад, зниження дихальної здатності),
- якщо ви страждаєте на захворювання, яке характеризується частими зупинками дихання під час сну, що може зробити вас дуже сонливим протягом дня (апное сну),
- якщо ви страждаєте на мікседему (зобоподібне захворювання, яке характеризується сухістю, холодом та набуханням шкіри, яке впливає на обличчя та кінцівки),
- якщо ваша щитоподібна залоза не виробляє достатньо гормонів (гіпотиреоз),
- якщо ваші надниркові залози не виробляють достатньо гормонів (надниркова недостатність або хвороба Аддісона),
- якщо ви страждаєте на психічні захворювання, які супроводжуються частковою втратою відчуття реальності (психоз), викликані алкоголізмом або інтоксикацією іншими речовинами (психоз, викликаний речовинами),
- якщо у вас є проблеми з жовчними камнями,
- якщо у вас є аномальне збільшення розміру простати (гіпертрофія простати),
- якщо ви страждаєте на алкоголізм або делірій тременс,
- якщо у вас є запалення підшлункової залози (панкреатит),
- якщо у вас є низький кров'яний тиск (гіпотензія),
- якщо у вас є високий кров'яний тиск (гіпертензія),
- якщо у вас є попереднє захворювання серцево-судинної системи,
- якщо у вас є черепно-мозкова травма (через ризик підвищення тиску в мозку),
- якщо ви страждаєте на епілепсію або схильні до конвульсій,
- якщо ви також приймаєте лікування інгібіторами МАО (які використовуються для лікування депресії, хвороби Паркінсона або бактеріальних інфекцій), наприклад, препаратами, які містять транілципромін, фенелзін, ізокарбоксазид, моклобемід і лінезолід,
- якщо ви відчуваєте сонливість або іноді раптово засинаєте.
Цей лікарський засіб може викликати проблеми з диханням під час сну. Ці проблеми можуть включати зупинки дихання під час сну, пробудження через нестачу повітря, труднощі з засинанням або надмірну сонливість вдень. Якщо ви або інша людина спостерігають ці симптоми, зверніться до вашого лікаря. Ваш лікар може вирішити зменшити дозу.
Повідомте вашому лікареві, якщо ви мали будь-які з цих захворювань в минулому. Повідомте також вашому лікареві, якщо ви маєте будь-яке з них під час лікування Таноналлою.
Найбільш серйозним наслідком передозування опіоїдів є депресія дихання(повільне та поверхневе дихання). Це також може викликати зниження концентрації кисню в крові, що може викликати втрату свідомості тощо.
Повідомте вашому лікареві, якщо ви страждаєте на рак, пов'язаний з перитонеальним метастазом або з кишковою непрохідністю на ранніх стадіях захворювання травної системи та тазу.
Зверніться до вашого лікаря, якщо у вас є сильний біль у верхній частині живота, який може поширитися на спину, нудота, блювота або гарячка, оскільки ці можуть бути симптомами, пов'язаними з запаленням підшлункової залози (панкреатит) або системи жовчних шляхів.
Якщо ви відчуваєте сильну діарею на початку лікування, це може бути пов'язано з дією налоксону. Це може бути ознакою того, що функція кишечника нормалізується. Ця діарея може виникнути протягом перших 3-5 днів лікування. Якщо вона триває після цього періоду або якщо вас це турбує, зверніться до вашого лікаря.
Якщо ви приймали інший опіоїд, ви можете відчувати симптоми абстиненції незабаром після початку лікування Таноналлою, наприклад, неспокій, напади поту та м'язовий біль. Якщо ви відчуваєте будь-які з цих симптомів, вам може знадобитися спеціальний контроль з боку вашого лікаря.
Як і інші опіоїди, оксикодон може впливати на нормальне вироблення гормонів в організмі, таких як кортизол або статеві гормони, особливо якщо приймаються високі дози протягом тривалого часу. Якщо ви відчуваєте тривалі симптоми, такі як нездужання (включно з блювотою), втрату апетиту, втому, слабкість, головокружіння, зміни в менструальному циклі, імпотенцію, зниження фертильності або зниження статевого потягу, проконсультуйтеся з вашим лікарем, щоб він міг контролювати ваші гормональні рівні.
Хірургічне втручання
Якщо вам потрібно піддатися хірургічному втручанню, повідомте лікарям, що ви приймаєте лікування Таноналлою.
Довгострокове лікування
Якщо ви приймаєте Таноналлу протягом тривалого часу, ви можете відчувати толерантність. Це означає, що вам потрібно буде більшої дози для досягнення бажаного зняття болю. Тривале використання Таноналли також може викликати фізичну залежність. Можуть виникнути симптоми абстиненції, якщо лікування раптово припинити (неспокій, напади поту, м'язовий біль). Якщо ви припиняєте лікування, вам потрібно буде поступово зменшувати добову дозу, проконсультувавшись з вашим лікарем.
Толерантність, залежність і звикання
Цей лікарський засіб містить оксикодон, який є опіоїдом, і може викликати залежність та/або звикання. |
Залежністьпсихологічна
Активна речовина гідрохлорид оксикодону без комбінування має ті самі характеристики зловживання, що й інші потужні опіоїди (потужні анальгетики). Це може викликати психологічну залежність. Лікарські засоби, які містять гідрохлорид оксикодону, повинні уникатися в пацієнтів, які мають або мали раніше історію зловживання алкоголем, наркотиками або лікарськими засобами.
Неправильне використання Таноналли
Ці таблетки не призначені для лікування синдрому абстиненції.
Таблетку потрібно ковтати цілою, не дроблячи, не розбиваючи, не жуючи та не подрібнюючи.
Дроблення, розбивання, жування або подрібнення таблеток може призвести до всмоктування летальної дози гідрохлориду оксикодону (див. розділ 3 «Якщо ви прийняли більше Таноналли, ніж потрібно»).
Ніколи не зловживайте Таноналлою, особливо якщо ви страждаєте на залежність від наркотиків. Якщо ви залежні від речовин, таких як героїн, морфін або метадон, ви можете відчувати сильні симптоми абстиненції, якщо неправильно використовуватимете Таноналлу, оскільки вона містить активну речовину налоксон. Це може погіршити існуючі симптоми абстиненції.
Також ніколи не розчиняйте ці таблетки для ін'єкції (наприклад, в кровоносний судину). Причина полягає в тому, що вони містять тальк, який може викликати руйнування місцевих тканин (некроз) та порушення легеневої тканини (легенева гранульома). Це зловживання також може мати інші серйозні наслідки та навіть викликати смерть.
Ви можете побачити залишки таблетки в ваших фекаліях. Не турбуйтеся, оскільки активні речовини (гідрохлорид оксикодону та гідрохлорид налоксону) вже були вивільнені раніше, коли таблетка проходила через шлунок та кишечник, і почала діяти в вашому організмі.
Допінг
Використання цього лікарського засобу може давати позитивні результати при тестах на допінг. Використання цього лікарського засобу як допінгу може загрожувати здоров'ю.
Діти та підлітки
Таноналла не була вивчена у дітей та підлітків молодше 18 років. Її безпека та ефективність не були доведені у дітей та підлітках. Тому не рекомендується її використання у дітей та підлітках молодше 18 років.
Інші лікарські засоби та Таноналла
Повідомте вашому лікареві або фармацевту, якщо ви приймаєте, нещодавно приймали або можете приймати будь-які інші лікарські засоби.
Відповідно з даними клінічних досліджень, прийом гідрохлориду оксикодону/гідрохлориду налоксону разом із седативними лікарськими засобами, такими як бензодіазепіни або пов'язані з ними лікарські засоби (лікарські засоби, які можуть впливати на функцію мозку, див. нижче), збільшує ризик сонливості, труднощів з диханням (депресія дихання), коми та може загрожувати життю. Через це спільне використання повинно розглядатися тільки тоді, коли інші варіанти лікування не можливі.
Однак, якщо ваш лікар призначає оксикодон/гідрохлорид налоксону разом із седативними лікарськими засобами, ваш лікар повинен обмежити дозу та тривалість спільного лікування.
Повідомте вашому лікареві про всі седативні лікарські засоби, які ви приймаєте, та слідуйте точно рекомендованим дозам, призначеним вашим лікарем. Це може бути корисно повідомити друзям або членам сім'ї, які знають про ознаки та симптоми, вказані вище. Зверніться до вашого лікаря, коли ви відчуваєте ці симптоми.
Деякі приклади лікарських засобів, які можуть впливати на функцію мозку, включають:
- інші потужні анальгетики (опіоїди),
- лікарські засоби для лікування епілепсії, болю та тривоги, такі як габапентин та прегабалін,
- соноліки та транквілізатори (седативні лікарські засоби, включно з бензодіазепінами, гіпнотиками, анксіолітиками),
- лікарські засоби для лікування депресії,
- лікарські засоби, які використовуються для лікування алергій, вертIGO або нудоти (антігістаміни або антиеметики),
- лікарські засоби, які використовуються для лікування психіатричних або психічних захворювань (фенотіазини, нейролептики, антипсихотики),
- м'язові релаксанти,
- лікарські засоби для лікування хвороби Паркінсона.
Ризик появи побічних ефектів збільшується, якщо використовуються антидепресанти (наприклад, циталопрам, дулоксетин, есциталопрам, флуоксетин, флуvoxamina, пароксетин, сертралін, венлафаксин). Ці лікарські засоби можуть взаємодіяти з оксикодоном та можуть викликати симптоми, такі як ритмічні та неповільні скорочення м'язів, включно з м'язами, які контролюють рухи очей, агітацію, надмірну потливість, тремор, гіперрефлексію, підвищення м'язового тонусу та температуру тіла вище 38 °C. Зверніться до вашого лікаря, якщо ви відчуваєте ці симптоми.
- Якщо ви приймаєте ці таблетки одночасно з іншими лікарськими засобами, ефекти таблеток або інших лікарських засобів, описаних нижче, можуть змінитися. Повідомте вашому лікареві, якщо ви приймаєте:
- лікарські засоби, які знижують здатність крові згортатися (походні кумарину), може збільшитися або зменшитися швидкість згортання,
- антібіотики макроліди (наприклад, кларитроміцин, еритроміцин або телітроміцин),
- протигрибкові засоби типу -азолів (наприклад, кетоконазол, вориконазол,ітраконазол або позаконазол),
- специфічний тип лікарського засобу, який використовується для лікування ВІЛ (інгібітори протеази, наприклад, ритонавір, індінавір, нелфінавір або саквінавір),
- циметидин (лікарський засіб для лікування виразкової хвороби шлунку, диспепсії або кислотності),
- рифампіцин (використовується для лікування туберкульозу),
- карбамазепін (використовується для лікування нападів або конвульсій та деяких болісних захворювань),
- фенітойн (використовується для лікування нападів або конвульсій).
- рослинний лікарський засіб, який називається трава Святого Івана (також відомий як Hypericum perforatum),
- хінідин (лікарський засіб для лікування аритмій).
Прийом Таноналли з їжею, напоями та алкоголем
Вживання алкоголю під час прийому Таноналли може зробити вас ще більш сонливим або збільшити ризик серйозних побічних ефектів, таких як поверхневе дихання з ризиком зупинки дихання, та втрату свідомості. Рекомендується уникати вживання алкоголю під час прийому Таноналли.
Ви повинні уникати вживання грейпфрутового соку під час прийому Таноналли.
Вагітність та лактація
Якщо ви вагітні або годуєте грудьми, вважаєте, що можете бути вагітною або плануєте вагітність, проконсультуйтеся з вашим лікарем або фармацевтом перед використанням цього лікарського засобу.
Вагітність
Під час вагітності використання цього лікарського засобу повинно бути обмежене за можливості. Якщо він використовується протягом тривалого періоду вагітності, гідрохлорид оксикодону може викликати симптоми абстиненції у новонародженого. Якщо гідрохлорид оксикодону вводиться під час пологів, новонароджений може відчувати депресію дихання (повільне та поверхневе дихання).
Грудне вигодовування
Грудне вигодовування повинно бути припинено під час лікування Таноналлою. Гідрохлорид оксикодону проникає в грудне молоко. Не відомо, чи проникає гідрохлорид налоксону в грудне молоко. Тому не можна виключити ризик для дитини, особливо якщо мати приймає кілька доз цього лікарського засобу.
Водіння транспортних засобів та використання машин
Таноналла може впливати на вашу здатність водити транспортні засоби та використовувати машини. Це відбувається особливо на початку лікування цим лікарським засобом, після збільшення дози або після зміни з іншого лікарського засобу. Однак, ці побічні ефекти зникають після встановлення дози цього лікарського засобу.
Таноналла асоціюється з сонливістю та епізодами раптового сну. Якщо ви відчуваєте ці побічні ефекти, не повинні водити транспортні засоби або використовувати машини. Якщо це відбувається з вами, повідомте вашому лікареві.
Спитайте вашого лікаря, чи можете ви водити транспортні засоби або використовувати машини під час лікування цим лікарським засобом.
Таноналла містить натрій
Цей лікарський засіб містить менше 1 ммоль натрію (23 мг) на пролонговану таблетку; це означає, що він практично «не містить натрію».
3. Як приймати Таноналлу
Слідкуйте точно інструкціям щодо застосування цього лікарського засобу, вказаним вашим лікарем. У разі сумнівів проконсультіруйтесь знову з вашим лікарем або фармацевтом.
Таноналла - це таблетка з тривалою дією, що означає, що активні речовини виділяються протягом тривалого періоду часу. Її дія триває 12 годин.
Якщо ваш лікар не вказав інше, звичайна доза така:
Для лікування болю
Дорослі
Звичайна початкова доза становить 10 мг гідрохлориду оксикодону/5 мг гідрохлориду налоксону в таблетках з тривалою дією кожні 12 годин.
Ваш лікар вирішить, яку дозу Таноналли ви повинні приймати на день і як розділити загальну добову дозу між дозою ранку і дозою вечора. Ваш лікар також вирішить, чи потрібно коригувати дозу під час лікування. Вашу дозу буде адаптовано до вашого ступеня болю та індивідуальної чутливості. Ви повинні отримувати мінімальну необхідну дозу для полегшення болю. Якщо ви вже приймали лікування опіоїдами, початкова доза Таноналли може бути вищою.
Максимальна добова доза становить 160 мг гідрохлориду оксикодону та 80 мг гідрохлориду налоксону. Якщо вам потрібно більша доза, ваш лікар може призначити вам більше гідрохлориду оксикодону без гідрохлориду налоксону. Однак максимальна добова доза гідрохлориду оксикодону не повинна перевищувати 400 мг. Корисний ефект гідрохлориду налоксону на кишкову діяльність може бути порушений, якщо збільшується доза гідрохлориду оксикодону без збільшення дози гідрохлориду налоксону.
Якщо ви заміняєте Таноналлу на інший опіоїдний анальгетик, ваша кишкова діяльність, ймовірно, погіршиться.
Якщо ви відчуваєте біль між прийомами Таноналли, вам може знадобитися анальгетик з швидкою дією. Таноналла не підходить у цьому випадку. Проконсультіруйтесь з вашим лікарем.
Якщо у вас є відчуття, що ефект Таноналли надто сильний або надто слабкий, проконсультіруйтесь з вашим лікарем або фармацевтом.
Вікова доза
Загалом не потрібно коригувати дозу у пацієнтів похилого віку з нормальною функцією нирок і/або печінки.
Порушення функції печінки або нирок
Якщо у вас є порушення функції нирок будь-якого ступеня або легке порушення функції печінки, ваш лікар призначить цей лікарський засіб з особливою обережністю. Якщо у вас є помірне або важке порушення функції печінки, не слід приймати Таноналлу (див. також розділ 2 "Не приймати Таноналлу" та "Попередження та обережність").
Форма застосування
Перорально.
- Прійміть Таноналлу з склянкою води.
Таблетку слід ковтати цілою, не розбиваючи, не роздираючи, не жуючись та не подрібнюючи.
- Ви можете приймати таблетки з тривалою дією з їжею або без неї.
Прійміть Таноналлу кожні 12 годин, дотримуючись постійного графіку (напр., о 8 годині ранку та о 8 годині вечора).
[Блістер, який можна відкрити:]
Як вийняти таблетки з блистерів, що резистентні до дітей
Таблетки упаковано в блистер однодозовий, резистентний до дітей.
Не тиснітьтаблетки крізьплівку блистеру.
Вийміть таблетки наступним чином:
- Згиніть алвеол уздовж перфорованої лінії.
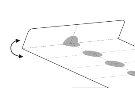
- Відокремте одну плівку блистеру за перфорованими лініями.
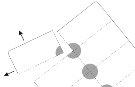
- Відкрійте плівку блистеру обережно з кута.
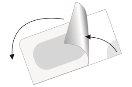
Вийміть таблетку.
[Блістер, який можна відкрити натисканням:]
Як вийняти таблетки з блистерів, що резистентні до дітей
Таблетки упаковано в блистер однодозовий, резистентний до дітей.
Для виїмання таблетки натисніть на неї крізь плівку блистеру, посилений.
Тривалість лікування
Загалом не слід приймати Таноналлу довше, ніж необхідно. Якщо ви приймаєте Таноналлу протягом тривалого часу, ваш лікар повинен регулярно перевіряти, чи продовжуєте ви її потребувати.
Якщо ви прийняли більше Таноналли, ніж потрібно
Якщо ви прийняли більше гідрохлориду оксикодону/гідрохлориду налоксону, ніж потрібно, негайно проконсультіруйтесь з вашим лікарем, фармацевтом або зателефонуйте в Токсикологічну службу, телефон: 91 562 04 20, вказавши лікарський засіб та кількість, що використовувалась.
Передозування може спричинити:
- зуження зіниць,
- повільне та поверхневе дихання (депресія дихання),
- сонливість до втрати свідомості,
- низький тонус м'язів (гіпотонія),
- зниження частоти серцевих скорочень, та
- зниження артеріального тиску.
У важких випадках можуть виникнути втрати свідомості (кома), нагромадження рідини в легенях та колапс кровообігу, що може бути смертельним у деяких випадках.
Ви повинні уникати ситуацій, які вимагають високого рівня уваги, наприклад, водіння автомобіля.
Якщо ви забули прийняти Таноналлу
Якщо ви забули прийняти Таноналлу або якщо ви прийняли меншу дозу, ніж призначена, ви можете не відчувати анальгетичного ефекту.
Якщо ви забули прийняти дозу, слідуйте наступним інструкціям:
- Якщо залишилося 8 годин або більше до наступної звичайної дози: прийміть забуту дозу негайно, та продовжуйте звичайний графік.
- Якщо залишилося менше 8 годин до наступної звичайної дози: прийміть забуту дозу. Чекайте ще 8 годин, перш ніж прийняти наступну дозу. Спробуйте відновити початковий графік (напр., о 8 годині ранку та о 8 годині вечора). Не прийміть Таноналлу більше одного разу протягом 8 годин.
Не прийміть подвійну дозу для компенсації забутих доз.
Якщо ви припинили лікування Таноналлою
Не припиняйте лікування Таноналлою без консультації з вашим лікарем.
Якщо вам не потрібно продовжувати лікування, ви повинні зменшити добову дозу поступово після обговорення з вашим лікарем. Таким чином, ви уникнете симптомів абстиненції, таких як неспокій, потіння та м'язовий біль.
Якщо у вас є будь-які інші питання щодо використання цього лікарського засобу, проконсультіруйтесь з вашим лікарем або фармацевтом.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може спричинити побічні ефекти, хоча не всі люди їх відчувають.
Важливі побічні ефекти, на які слід звернути увагу, та що робити, якщо вони виникають
Якщо ви вже маєте один з наступних важливих побічних ефектів, негайно проконсультіруйтесь з найближчим лікарем.
Повільне та поверхневе дихання (депресія дихання) - це основна небезпека передозування опіоїдів. Це відбувається переважно у пацієнтів похилого віку та ослаблених. Опіоїди також можуть спричинити сильне зниження артеріального тиску у чутливих пацієнтів.
Наступні побічні ефекти були спостережені у пацієнтів, які приймають лікування болю:
Часті(може впливати до 1 з 10 осіб)
- зниження або втрата апетиту,
- важкість із сном, втома або слабкість,
- чуття головокружіння або "кружіння всього", біль у голові, сонливість,
- пітливість,
- біль у животі, запор, діарея, сухість у роті, диспепсія, нудота, нездужання, метеоризм,
- свербіння шкіри, шкірні реакції, збільшення пітливості,
- чуття незвичайної слабкості.
Нечасті(може впливати до 1 з 100 осіб)
- алергічні реакції,
- зниження статевого потягу,
- незпокій, дивні думки, тривога, плутання, депресія, нервозність,
- епілептичні припадки (особливо у осіб з епілептичними розладами або схильністю до конвульсій), труднощі з концентрацією уваги, порушення смаку, порушення мови, омана, тремор, втрата енергії.
- порушення зору,
- чуття стискання в грудній клітці, особливо якщо ви вже маєте захворювання коронарних судин, серцебиття,
- зниження артеріального тиску, збільшення артеріального тиску,
- важкість із диханням, ринорея, кашель,
- розтягнення живота,
- збільшення печінкових ферментів, печінкова коліка,
- м'язові спазми, м'язові скорочення, м'язовий біль,
- збільшення потреби в сечовипусканні,
- симптоми абстиненції, такі як неспокій,
- грудний біль,
- озноб, чуття нездужання, біль, спрага,
- набухання рук, гомілок або ніг,
- втрата ваги,
- пошкодження через нещасні випадки.
Рідкі(може впливати до 1 з 1000 осіб)
- збільшення частоти серцевих скорочень,
- залежність від лікарського засобу,
- зевання,
- стоматологічні порушення,
- збільшення ваги.
Частота невідома(не може бути оцінена на основі доступних даних)
- еуфорія, галюцинації, кошмари, агресія,
- поколювання, сильна сонливість,
- поверхневе дихання,
- ерукти,
- важкість із сечовипусканням,
- імпотенція,
- проблеми з диханням під час сну (апное сну).
Відомо, що активна речовина гідрохлорид оксикодону, якщо не поєднується з гідрохлоридом налоксону, має наступні побічні ефекти, які відрізняються від перелічених:
Оксикодон може спричинити респіраторні проблеми (депресія дихання), зниження розміру зіниць, спазми м'язів бронхів та гладких м'язів, а також депресію кашлевого рефлексу.
Часті(може впливати до 1 з 10 осіб)
- порушення настрою та зміни особистості (напр., депресія, чуття надмірної щастя), зниження діяльності, збільшення діяльності,
- гіпо,
- важкість із сечовипусканням.
Нечасті(може впливати до 1 з 100 осіб)
- дегідратация,
- незпокій, порушення сприйняття (напр., галюцинації, дезорієнтація),
- важкість із концентрацією уваги, мігрень, збільшення м'язового тонусу, м'язові скорочення, зниження чутливості до болю або дотику, порушення координації,
- слухові порушення,
- розширення судин,
- порушення голосу (дисфонія),
- важкість із ковтанням,
- стан, при якому кишка перестає функціонувати правильно (ілей), виразки у роті, підrážнення ясен,
- сухість шкіри,
- зниження рівня статевих гормонів, яке може впливати на виробництво сперми у чоловіків або менструальний цикл у жінок
- набухання через утримання води, толерантність до лікарського засобу.
Рідкі(може впливати до 1 з 1000 осіб)
- інфекції, такі як герпес у роті або герпес (що може спричинити пухирі навколо рота або генітальної області),
- збільшення апетиту,
- чорний кал (з вигляду смоли), кровотеча десен,
- свербіжна висипка (уртикарія).
Частота невідома(не може бути оцінена на основі доступних даних)
- гострі алергічні реакції (анafilактичні реакції),
- збільшення чутливості до болю,
- карієс зубів,
- проблеми з потоком жовчі, проблема, що впливає на одну з клапанів у кишці, що може спричинити сильний біль у верхній частині живота (дисфункція сфінктера Одді),
- відсутність менструації,
- синдром абстиненції у новонародженого.
Звіт про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, проконсультіруйтесь з вашим лікарем або фармацевтом, навіть якщо це можливі побічні ефекти, які не перелічені в цьому листку. Ви також можете повідомити про них безпосередньо через Систему фармакологічного нагляду за лікарськими засобами для людини: https://www.notificaram.es. Надсилаючи повідомлення про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Зберігання Таноналли
Тримайте цей лікарський засіб поза зоною досяжності дітей.
Не використовувати цей лікарський засіб після закінчення терміну придатності, вказаного на упаковці, пляшці або блистеру після CAD/EXP. Термін придатності - останній день місяця, який вказано.
Блістер:
Не зберігайте при температурі вище 25°C.
Пляшки:
Не зберігайте при температурі вище 30°C.
Термін придатності після першого відкриття: 3 місяці.
Лікарські засоби не повинні викидатися у водопровідні труби або сміття. Відкладайте упаковки та лікарські засоби, які вам не потрібні, у пункті збору фармацевтичних продуктів. У разі сумнівів проконсультіруйтесь з вашим фармацевтом, як позбутися упаковок та лікарських засобів, які вам не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад Таноналли
Активними речовинами є гідрохлорид оксикодону та гідрохлорид налоксону.
Кожна таблетка з тривалою дією містить 5 мг гідрохлориду оксикодону (еквівалентно 4,5 мг оксикодону) та 2,5 мг гідрохлориду налоксону (як 2,74 мг гідрохлориду налоксону дигідрату, еквівалентно 2,25 мг налоксону).
Інші допоміжні речовини:
Ядро таблетки:
Ацетат полівінілу
Повідон К30
Лаурілсульфат натрію
Колоїдна кремнеземна діоксид
Мікрокристалічна целюлоза
Стеарат магнію
Покриття таблетки:
Полівініловий спирт
Діоксид титану (E171)
Макрогол 3350
Тальк
Вигляд продукту та вміст упаковки
Таблетка з тривалою дією біла, кругла, двовигнута з діаметром 4,7 мм та товщиною 2,9-3,9 мм.
Таноналла доступна у блистерах однодозових, резистентних до дітей, які містять 10x1 (госпітальна упаковка), 14x1, 20x1, 28x1, 30x1, 50x1, 56x1, 60x1, 98x1 та 100x1 таблеток з тривалою дією або
блістері, резистентному до дітей, який містить 28, 56 та 84 таблетки з тривалою дією або у пляшках HDPE з кришкою, резистентною до дітей, які містять 50 та 100 таблеток з тривалою дією.
Можливо, не всі розміри упаковок будуть реалізовані.
Власник дозволу на маркетинг та відповідальна особа за виробництво
Власник дозволу на маркетинг
Sandoz Farmacéutica, S.A.
Центр підприємництва Парке Норте
Будинок Робле
Вулиця Серрано Гальваче, 56
28033 Мадрид
Іспанія
Відповідальна особа за виробництво
Develco Pharma GmbH
Грінматт 27, Баден-Вюртемберг
Д-79650 Шопфгайм
Німеччина
Salutas Pharma GmbH
Отто-фон-Геріке-Алле 1
39179 Барлебен
Німеччина
Цей лікарський засіб дозволено в державах-членах Європейського економічного простору та у Великій Британії (Північна Ірландія) під наступними назвами:
Німеччина: Oxycodon-HCl/Naloxon-HCl HEXAL 5 мг/2,5 мг Retardtabletten
Словаччина: Oxycodone/Naloxone Sandoz 5 мг/2,5 мг
Словенія: Oksikodon/nalokson Lek 5 мг/2,5 мг таблетки з подовженим вивільненням
Велика Британія: Doneloxon 5 мг/2,5 мг Пrolonged-release таблетки
Фінляндія: Tanonalla 5 мг/2,5 мг депо-таблиця
Ірландія: Dancex SR Plus 5 мг/2,5 мг Пrolonged-release таблетки
Італія: Ossicodone e Naloxone Sandoz
Чехія: Oxycodon/Naloxon Sandoz 5 мг/2,5 мг
Швеція: Oxycodone/Naloxone Sandoz 5 мг/2,5 мг депо-таблиця
Норвегія: Tanonalla 5 мг/2,5 мг депо-таблиця
Ісландія: Tanonalla 5 мг/2,5 мг фордатафла
Польща: Xanconalon
Португалія: Oxicodona + Naloxona Sandoz 10мг/5мг компреси з плівковим покриттям
Дата останнього перегляду цього листка:травень 2025 року.
Детальна інформація про цей лікарський засіб доступна на сайті Іспанського агентства лікарських засобів та медичних продуктів (AEMPS) http://www.aemps.gob.es/

Скільки коштує ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ в Іспанії у 2025 році?
ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ коштує в середньому 12.69 євро у грудень, 2025 році. Ціна може змінюватися залежно від регіону, аптеки та наявності рецепта. Рекомендуємо перевіряти актуальну вартість у місцевих аптеках або через онлайн-сервіси.
- Країна реєстрації
- Середня ціна в аптеках12.69 EUR
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇФорма випуску: ТАБЛЕТКА З МОДИФІКОВАНИМ ВИВІЛЬНЕННЯМ, 10 мг/5 мгДіючі речовини: oxycodone and naloxoneВиробник: Neuraxpharm Spain S.L.Потрібен рецептФорма випуску: ТАБЛЕТКА З МОДИФІКОВАНИМ ВИВІЛЬНЕННЯМ, 20 мг/10 мгДіючі речовини: oxycodone and naloxoneВиробник: Neuraxpharm Spain S.L.Потрібен рецептФорма випуску: ТАБЛЕТКА З МОДИФІКОВАНИМ ВИВІЛЬНЕННЯМ, 30 мг/15 мгДіючі речовини: oxycodone and naloxoneВиробник: Neuraxpharm Spain S.L.Потрібен рецепт
Аналоги ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ у Poland
Лікарі онлайн щодо ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ТАНОНАЛЛА 5 мг/2,5 мг ТАБЛЕТКИ ПРОДОВЖЕНОЇ ДІЇ – за рішенням лікаря та згідно з місцевими правилами.














