

РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУ

Запитайте лікаря про рецепт на РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУ

Інструкція із застосування РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУ
Введення
Опис препарату: інформація для пацієнта
Реватіо 10 мг/мл порошок для пероральної суспензії
сілденафіл
Всім пацієнтам потрібно уважно прочитати цю брошуру перед початком прийому цього ліків, оскільки вона містить важливу інформацію для вас.
- Збережіть цю брошуру, оскільки вам може знадобитися знову її прочитати.
- Якщо у вас виникли питання, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей препарат призначений тільки для вас, і його не можна давати іншим людям, навіть якщо вони мають相同ні симптоми, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не вказані в цій брошурі. Див. розділ 4.
Зміст брошури
- Що таке Реватіо і для чого він використовується
- Що потрібно знати перед початком прийому Реватіо
- Як приймати Реватіо
- Можливі побічні ефекти
- Збереження Реватіо
- Зміст упаковки та додаткова інформація
1. Що таке Реватіо і для чого він використовується
Реватіо містить активну речовину сілденафіл, який належить до групи лікарських засобів, званих інгібіторами фосфодіестерази типу 5 (ПДЕ5).
Реватіо знижує артеріальний тиск у легенях, розширюючи кровоносні судини легень. Реватіо використовується для лікування підвищеного артеріального тиску у легенях (гіпертензія легеневої артерії) у дорослих і дітей та підлітків у віці від 1 до 17 років.
2. Що потрібно знати перед початком прийому Реватіо
Не приймати Реватіо
- якщо ви алергічні на сілденафіл або будь-який інший компонент цього лікарського засобу (перелічені в розділі 6).
- якщо ви приймаєте лікарські засоби, які містять нітрати або донори оксиду азоту, такі як нітрит амілу ( «поппери»). Ці лікарські засоби часто використовуються для полегшення болю в грудній клітці (або стенокардії). Реватіо може підвищити дію цих лікарських засобів. Ви повинні повідомити свого лікаря, якщо приймаєте будь-який з цих лікарських засобів. Якщо ви не впевнені, проконсультуйтеся з вашим лікарем або фармацевтом.
- якщо ви приймаєте ріоцігуат. Цей лікарський засіб використовується для лікування гіпертензії легеневої артерії (тобто підвищеного тиску в легенях) та хронічної тромбоемболічної гіпертензії легеневої артерії (тобто підвищеного тиску в легенях, викликаного тромбами). Інгібітори ПДЕ5, такі як Реватіо, показали, що підвищують гіпотензивну дію цього лікарського засобу. Якщо ви приймаєте ріоцігуат або не впевнені, проконсультуйтеся з вашим лікарем.
- якщо ви нещодавно мали інсульт, інфаркт міокарда або якщо у вас є важка хвороба печінки або дуже низький артеріальний тиск (<90/50 мм рт. ст.).
- якщо ви приймаєте лікарські засоби для лікування грибкових інфекцій, таких як кетоконазол або ітраконазол, або лікарські засоби, які містять ритонавір (для СНІДу).
- якщо ви раніше мали втрату зору через проблему з кровотоком у нерві ока, звану неартеріальною передньою ішемічною нейропатією зору (НОІНЗ).
Попередження та обережність
Проконсультуйтеся з вашим лікарем або фармацевтом перед початком прийому Реватіо, якщо:
- у вас є хвороба, викликана блокуванням або звуженням вени в легенях замість блокування або звуження артерії.
- у вас є важка хвороба серця.
- у вас є проблема з камерами серця.
- у вас є підвищений артеріальний тиск у легенях.
- у вас є низький артеріальний тиск у стані спокою.
- ви втрачаєте велику кількість рідини (дегідратاسیون), яка може виникнути, коли ви потієте сильно або не п'єте достатньо рідини. Це може статися, якщо ви хворієте на гарячку, блювоту або діарею.
- у вас є рідкісна спадкова очна хвороба (пігментний ретиніт).
- у вас є аномалія червоних кров'яних тілець (серпоподібноклітинна анемія), рак крові (лейкемія), рак кістового мозку (мієлома) або будь-яка інша хвороба чи деформація пеніса.
- у вас є виразка шлунка або кровотеча (така як гемофілія) або носова кровотеча.
- ви використовуєте лікарські засоби для лікування еректильної дисфункції.
Коли інгібітори ПДЕ5, включаючи сілденафіл, використовуються для лікування еректильної дисфункції (ЕД), повідомлялися наступні побічні ефекти зору з невідомою частотою: зниження або втрата зору, тимчасова або постійна, одного або обох очей. Якщо ви відчуваєте зниження або втрату зору, перестаньте приймати Реватіо і негайно проконсультуйтеся з вашим лікарем(див. також розділ 4).
Відбулися тривалі та іноді болісні ерекції у чоловіків, які приймали сілденафіл. Якщо у вас тривала ерекція, яка триває більше 4 годин, перестаньте приймати Реватіо і негайно проконсультуйтеся з вашим лікарем(див. також розділ 4).
Особливі обережності у пацієнтів з проблемами нирок або печінки
Ви повинні повідомити свого лікаря, якщо у вас є проблеми з нирками або печінкою, оскільки може знадобитися корекція дози.
Діти
Реватіо не повинен прийматися дітям молодшим за 1 рік.
Прийом Реватіо з іншими лікарськими засобами
Повідомте свого лікаря або фармацевта, якщо ви використовуєте, нещодавно використовували або можете використовувати будь-який інший лікарський засіб.
- Лікарські засоби, які містять нітрати або донори оксиду азоту, такі як нітрит амілу ( «поппери»). Ці лікарські засоби часто використовуються для полегшення болю в грудній клітці або стенокардії (див. розділ 2. Перед початком прийому Реватіо).
- Повідомте свого лікаря або фармацевта, якщо ви приймаєте ріоцігуат.
- Лікарські засоби для лікування гіпертензії легеневої артерії (наприклад, бозентан, ілопрост).
- Лікарські засоби, які містять траву Святого Івана (лікарська рослина), рифампіцин (використовується для лікування бактеріальних інфекцій), карбамазепін, фенітойн і фенобарбітал (використовуються, серед іншого, для лікування епілепсії).
- Лікарські засоби, які інгібують згортання крові (наприклад, варфарин), хоча вони не викликали жодних побічних ефектів.
- Лікарські засоби, які містять еритроміцин, кларитроміцин, телітроміцин (антібіотики, які використовуються для лікування певних бактеріальних інфекцій), саквінавір (для СНІДу) або нефазодон (для депресії), оскільки може знадобитися корекція дози.
- Терápія з альфа-блокаторами (наприклад, доксазосином) для лікування гіпертензії або проблем з простатою, оскільки поєднання цих лікарських засобів може викликати симптоми зниження артеріального тиску (наприклад, головокружіння,失 сознания).
- Лікарські засоби, які містять сакубітріл/валсартан, які використовуються для лікування серцевої недостатності.
Прийом Реватіо з їжею та напоями
Не слід приймати грейпфрутовий сік під час лікування Реватіо.
Вагітність та годування грудьми
Якщо ви вагітні або годуєте грудьми, вважаєте, що можете бути вагітною або плануєте вагітність, проконсультуйтеся з вашим лікарем або фармацевтом перед прийомом цього лікарського засобу. Реватіо не повинен використовуватися під час вагітності, якщо це не абсолютно необхідно.
Реватіо не повинен прийматися жінками у фертильному віці, якщо вони не використовують відповідні методи контрацепції.
Реватіо проникає в грудне молоко у дуже низьких концентраціях і не очікується, що він нашкодить вашому дитяті.
Водіння транспортних засобів та використання машин
Реватіо може викликати головокружіння та вплинути на зір. Ви повинні знати, як ви реагуєте на цей лікарський засіб, перш ніж водити транспортні засоби або використовувати машини.
Реватіо містить сорбітол
Реватіо 10 мг/мл порошок для пероральної суспензії містить 250 мг сорбітолу на мл реконституїрованої суспензії.
Сорбітол є джерелом фруктози. Якщо ваш лікар сказав вам (або вашій дитині), що ви (або ваша дитина) маєте непереносимість певних цукрів, або якщо вам (або вашій дитині) діагностували спадкову непереносимість фруктози (СНФ), рідкісну генетичну хворобу, при якій пацієнт не може розщеплювати фруктозу, проконсультуйтеся з вашим лікарем перед прийомом цього лікарського засобу.
Реватіо містить бензоат натрію
Реватіо 10 мг/мл порошок для пероральної суспензії містить 1 мг бензоату натрію на мл реконституїрованої суспензії. Бензоат натрію може підвищити рівень певної речовини, званої білірубіном. Високі рівні білірубіну можуть викликати жовтяницю (жовтуватий колір шкіри та очей) та також можуть викликати ушкодження мозку (енцефалопатію) у новонароджених (до 4 тижнів життя).
Реватіо містить натрій
Реватіо 10 мг/мл порошок для пероральної суспензії містить менше 1 ммоль натрію (23 мг) на мл реконституїрованої суспензії; це означає, що він практично «не містить натрію».
3. Як приймати Реватіо
Слідувати точно інструкціям з прийому цього лікарського засобу, вказаним вашим лікарем.
У разі сумнівів проконсультуйтеся з вашим лікарем або фармацевтом.
Для дорослих рекомендується доза 20 мг тричі на добу (прийом через 6-8 годин) з або без їжі.
Використання у дітей та підлітків
Для дітей та підлітків у віці від 1 до 17 років рекомендується доза 10 мг (1 мл пероральної суспензії) тричі на добу для дітей та підлітків, які важать 20 кг або менше, або 20 мг (2 мл пероральної суспензії) тричі на добу для дітей та підлітків, які важать більше 20 кг, приймається з або без їжі. У дітей не слід використовувати вищі дози, ніж рекомендовані.
Пероральну суспензію потрібно добре перемішати протягом мінімум 10 секунд перед використанням.
Інструкції з реконституції пероральної суспензії
Рекомендується, щоб ваш фармацевт реконституївав (підготував) пероральну суспензію перед тим, як її буде використано.
Коли реконституїється пероральна суспензія, вона є рідиною. Якщо не реконституїровано порошок, потрібно реконституївати пероральну суспензію, слідуючи наступним інструкціям.
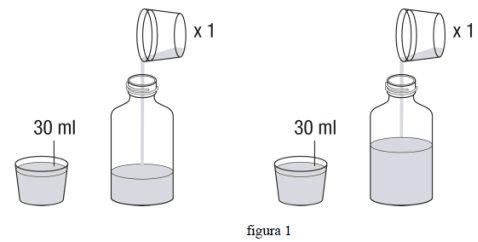
Примітка:Незалежно від дози, яку ви приймаєте, потрібно використовувати загальний об'єм 90 мл (3 х 30 мл) води для реконституції вмісту флакона.
- Ніжно стукайте по флакону, щоб звільнити порошок.
- Зніміть кришку.
- Виміряйте 30 мл води, наповнив мірний стакан (включений до комплекту) до позначки, і додайте до флакона. Виміряйте ще 30 мл води, використовуючи мірний стакан, і додайте до флакона. (фіг. 1)
- Знову закрийте кришку і потрясіть з силою протягом мінімум 30 секунд. (фіг. 2)
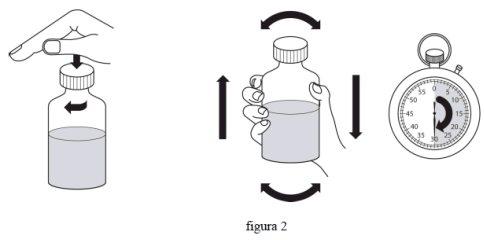
- Зніміть кришку.
- Використовуючи мірний стакан, додайте ще 30 мл води до флакона. Звжди потрібно додати загальний об'єм 90 мл (3 х 30 мл) води, незалежно від дози, яку ви приймаєте. (фіг. 3)
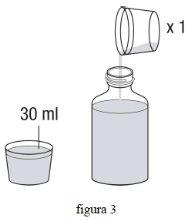
- Знову закрийте кришку і потрясіть флакон з силою протягом мінімум 30 секунд. (фіг. 4)
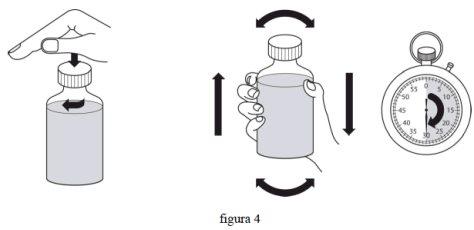
- Зніміть кришку.
- Вставте під тиск адаптер до горлечка флакона (як показано на фіг. 5 нижче). Надано адаптер, щоб ви могли наповнити оральну дозуючу шприцю ліками з флакона. Знову закрийте кришку.
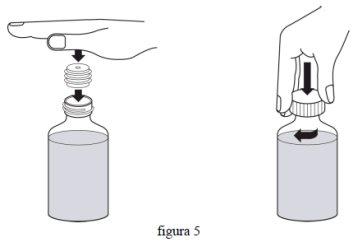
- Напишіть дату закінчення терміну зберігання реконституїрованої суспензії на етикетці флакона (термін зберігання реконституїрованої суспензії становить 30 днів з дати реконституції). Після цієї дати не використану суспензію потрібно видалити або повернути вашому фармацевту
Інструкції з використання
Ваш фармацевт покаже вам, як виміряти лікарський засіб за допомогою оральної дозуючої шприці, включеної до комплекту. Після реконституції пероральної суспензії її потрібно приймати лише за допомогою оральної дозуючої шприці, включеної до кожного комплекту. Дивіться наступні інструкції перед використанням суспензії.
- Потрясіть сильно флакон з реконституїрованою суспензією, закритий, протягом мінімум 10 секунд перед використанням. Зніміть кришку. (фіг. 6)
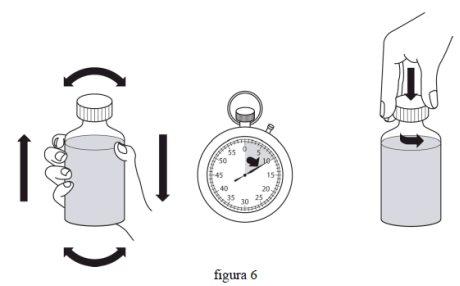
- З флаконом у вертикальному положенні на плоскій поверхні вставте наконечник оральної дозуючої шприці до адаптера. (фіг. 7)
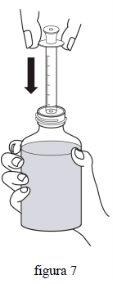
- Переверніть флакон, тримаючи оральну дозуючу шприцю на місці. Повільно витягніть поршень шприці до позначки, яка вказує вашу дозу (прийом 1 мл дає дозу 10 мг, прийом 2 мл дає дозу 20 мг). Для точного виміру дози верхній край поршня повинен бути вирівняний з відповідною позначкою на оральній дозуючій шприці. (фіг. 8)
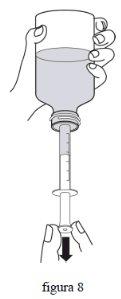
- Якщо ви бачите великі бульбашки, повільно натисніть поршень шприці всередину. Це знову введе лікарський засіб до флакона. Повторіть цей крок 3 рази.
- Поверніть флакон у вертикальне положення з оральною дозуючою шприцею, все ще на місці. Видаліть оральну дозуючу шприцю з флакона.
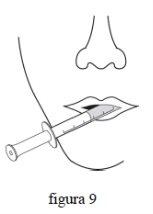
- Вставте наконечник оральної дозуючої шприці до рота. Направьте наконечник шприці всередину щоки. Повільно натисніть поршень шприці. Не виштовхуйте лікарський засіб швидко. Якщо лікарський засіб вводиться дитині, переконайтеся, що дитина сидить або знаходиться в положенні на боці перед введенням лікарського засобу. (фіг. 9)
- Знову закрийте кришку флакона, залишивши адаптер флакона на місці. Вимийте оральну дозуючу шприцю, як показано нижче.
Очищення і зберігання шприці:
- Після кожного використання вимийте шприцю. Вийміть поршень з шприці і вимийте обидві частини водою.
- Висушіть обидві частини. Вставте поршень до шприці. Зберігайте в чистому і безпечному місці разом з лікарським засобом.
Якщо ви прийняли більше Реватіо, ніж потрібно
Не слід приймати більше лікарського засобу, ніж вказав ваш лікар.
Якщо ви прийняли більше лікарського засобу, ніж рекомендовано, негайно проконсультуйтеся з вашим лікарем.
Прийом більше Реватіо, ніж потрібно, може підвищити ризик відомих побічних ефектів.
Якщо ви забули прийняти Реватіо
Якщо ви забули прийняти Реватіо, прийміть дозу як тільки ви згадаєте про це і продовжуйте приймати лікарський засіб означений час. Не прийміть подвійну дозу для компенсації пропущених доз.
Якщо ви припинили лікування Реватіо
Раптове припинення лікування Реватіо може привести до погіршення вашого стану. Не припиняйте приймати Реватіо, якщо тільки ваш лікар не порадить вам це зробити. Ваш лікар покаже вам, як зменшити дозу протягом кількох днів перед повним припиненням лікування.
Якщо у вас є будь-які інші питання щодо використання цього лікарського засобу, проконсультуйтеся з вашим лікарем або фармацевтом.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може викликати побічні ефекти, хоча не всі люди їх відчувають.
Якщо ви відчуваєте будь-який з наступних побічних ефектів, припиніть приймати Реватіо та повідомте про це своєму лікареві негайно (див. також розділ 2):
- якщо ви відчуваєте раптове зниження або втрату зору (частота невідома)
- якщо у вас є ерекція, яка триває безперервно більше 4 годин. Згідно повідомлень, після прийому силденафілу у чоловіків спостерігалися тривалі та іноді болісні ерекції (частота невідома)
Дорослі
Побічні ефекти, які повідомлялися дуже часто (можуть впливати на більше 1 з 10 пацієнтів), були: головний біль або червоність обличчя, диспепсія, діарея та біль у руках і ногах.
Побічні ефекти, які повідомлялися часто (можуть впливати до 1 з 10 пацієнтів), це: інфекція під шкірою, симптоми типу грипу, запалення носових синусів, зниження кількості червоних кров'яних тілець (анемія), затримка рідини, труднощі зі сном, тривога, мігрень, тремор, відчуття поколювання, відчуття паління, зниження чутливості, кровотеча з задньої частини ока, порушення зору, розмитість зору та чутливість до світла, ефекти на сприйняття кольорів, подразнення очей, червоні очі, вертіго, бронхіт, носова кровотеча, ринорея, кашель, закладення носа, запалення шлунка, гастроентерит, паління, геморой, розтягнення живота, сухість у роті, випадіння волосся, червоність шкіри, нічні поти, м'язовий біль, біль у спині та підвищення температури тіла.
Побічні ефекти, які повідомлялися рідко (можуть впливати до 1 з 100 пацієнтів), включали: зниження гостроти зору, подвійне бачення, незвичайне відчуття в оці, кровотеча з пеніса, наявність крові в сечі та/або семені та збільшення грудей у чоловіків.
Також повідомлялися висипання на шкірі, зниження або раптова втрата слуху та зниження артеріального тиску з невідомою частотою (частота не може бути оцінена за наявними даними).
Діти та підлітки
Побічні ефекти, які повідомлялися часто (можуть впливати до 1 з 10 пацієнтів), були: пневмонія, правша недостатність серця, кардіогенний шок, підвищення артеріального тиску в легенях, біль у грудній клітці, головокружіння, респіраторні інфекції, бронхіт, вірусна інфекція шлунка та кишечника, інфекції сечовидільної системи та перфорації зубів.
Наступні серйозні побічні ефекти вважалися пов'язаними з лікуванням і повідомлялися рідко (можуть впливати до 1 з 100 пацієнтів): алергічна реакція (як висипання на шкірі, запалення обличчя, губ, язика, чхання, труднощі з диханням або ковтанням), судоми, нерегулярні серцеві скорочення, порушення слуху, задишка, запалення травного тракту та чхання через порушення повітряного потоку.
Побічні ефекти, які повідомлялися дуже часто (можуть впливати на більше 1 з 10 пацієнтів), були: головний біль, блювота, інфекція горла, гарячка, діарея, грип та носова кровотеча.
Побічні ефекти, які повідомлялися часто (можуть впливати до 1 з 10 пацієнтів), були: нудота, збільшення ерекції, пневмонія та ринорея.
Повідомлення про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, проконсультуйтеся з лікарем або фармацевтом, навіть якщо це можливі побічні ефекти, які не перелічені в цьому листку. Ви також можете повідомити про них безпосередньо через Іспанську систему моніторингу лікарських засобів для людини: www.notificaram.es. Повідомляючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Зберігання Реватіо
Тримайте цей лікарський засіб поза зоною видимості та досягнення дітей.
Не використовуйте цей лікарський засіб після закінчення терміну придатності, вказаного на пляшці після CAD. Термін придатності - останній день місяця, який вказано.
Порошок
Не зберігайте при температурі вище 30°C.
Зберігайте в оригінальній упаковці для захисту від вологи.
Пероральна суспензія після відновлення
Зберігайте при температурі нижче 30°C або в холодильнику між 2°C та 8°C. Не заморожуйте. Через 30 днів після відновлення викиньте будь-які залишки пероральної суспензії.
Лікарські засоби не повинні викидатися у водопровідні труби чи у сміття. Спитайте у фармацевта, як позбутися упаковок та лікарських засобів, які вам більше не потрібні. Таким чином, ви допоможете захистити довкілля.
6. Зміст упаковки та додаткова інформація
Склад Реватіо
- Активний інгредієнт - силденафіл (у вигляді цитрату силденафілу).
Після відновлення кожен мл пероральної суспензії містить 10 мг силденафілу (у вигляді цитрату). Пляшка пероральної суспензії після відновлення (112 мл) містить 1,12 г силденафілу (у вигляді цитрату).
- Інші складові частини: Порошок для пероральної суспензії: сорбітол (Е240) (див. розділ 2 "Реватіо містить сорбітол"), ангідрид цитринової кислоти, сукралоза, цитрат натрію (Е331) (див. розділ 2 "Реватіо містить натрій"), ксантанову камедь, діоксид титану (Е171), бензоат натрію (Е211) (див. розділ 2 "Реватіо містить бензоат натрію" та "Реватіо містить натрій") та безводний колоїдний силікат; Ароматизатор винограду: мальтодекстрин, концентрат соку винограду, камедь акації, концентрат соку ананаса, ангідрид цитринової кислоти, натуральні ароматизатори.
Вигляд Реватіо та зміст упаковки
Реватіо випускається у вигляді білого або білуватого порошку для пероральної суспензії, який після відновлення з водою утворює білу пероральну суспензію з ароматом винограду.
Пляшка з коричневого скла об'ємом 125 мл (з навинчуваною кришкою з поліпропілену) містить 32,27 г порошку для пероральної суспензії.
Після відновлення пляшка містить 112 мл пероральної суспензії, з яких 90 мл використовуються для введення доз.
Форма випуску: 1 пляшка.
Кожна упаковка також містить мірний стаканчик з поліпропілену (градуйований для маркування 30 мл), орального дозувального шприца з поліпропілену (3 мл) з поршнем з високомолекулярного поліетилену та прес-адаптер для пляшки з низької щільності поліетилену.
Власник дозволу на розміщення лікарського засобу на ринку та відповідальна особа за виробництво
Власник дозволу на розміщення лікарського засобу на ринку:
Upjohn EESV, Rivium Westlaan 142, 2909 LD Capelle aan den IJssel, Нідерланди.
Виробник:
Fareva Amboise, Zone Industrielle, 29 route des Industries, 37530 Pocé-sur-Cisse, Франція
Mylan Hungary Kft., Mylan utca 1, Комаром, 2900, Угорщина.
Ви можете запитати додаткову інформацію про цей лікарський засіб, звернувшись до місцевого представника власника дозволу на розміщення лікарського засобу на ринку:
Іспанія
Viatris Pharmaceuticals, S.L.U.
Телефон: +34 900 102 712
Дата останнього перегляду цього листка:
Інші джерела інформації
Детальна інформація про цей лікарський засіб доступна на сайті Європейського агентства з лікарських засобів: http://www.ema.europa.eu. Також існують посилання на інші веб-сайти про рідкісні захворювання та орфанні лікарські засоби.
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУФорма випуску: ТАБЛЕТКА, 25 мгДіючі речовини: sildenafilВиробник: Adamed Pharma S.A.Потрібен рецептФорма випуску: ТАБЛЕТКА ЖУВАЛЬНА, 100 мгДіючі речовини: sildenafilВиробник: Farmalider S.A.Потрібен рецептФорма випуску: ТАБЛЕТКА, ЩО ЖУЄТЬСЯ, 25 мгДіючі речовини: sildenafilВиробник: Farmalider S.A.Потрібен рецепт
Аналоги РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУ у Польша
Аналог РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУ у Украина
Лікарі онлайн щодо РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на РЕВАТІО 10 мг/мл ПОРОШОК ДЛЯ ПРИГОТУВАННЯ ОРАЛЬНОГО РОЗЧИНУ – за рішенням лікаря та згідно з місцевими правилами.







