
REANDRON 1000 mg/4 ml SOLUCION INYECTABLE

Cómo usar REANDRON 1000 mg/4 ml SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
Reandron 1000mg / 4ml solución inyectable
Testosterona, undecanoato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamentoporque contiene información importante para usted.
|
Contenido del prospecto
- Qué es Reandron y para qué se utiliza
- Qué necesita saber antes de que le administren Reandron
- Cómo usar Reandron
- Posibles efectos adversos
- Conservación de Reandron
- Contenido del envase e información adicional
1. Qué es Reandron y para qué se utiliza
Reandron contiene testosterona, una hormona masculina, como principio activo.
Reandron se administra mediante inyección intramuscular; el medicamento administrado va liberándose con el tiempo.
Reandron se utiliza en hombres adultos para el tratamiento de sustitución de la testosterona y así tratar diversos problemas de salud derivados de la falta de testosterona (hipogonadismo masculino). Esto se debe confirmar mediante dos determinaciones separadas de testosterona en sangre y además presentar síntomas clínicos como por ejemplo:
- impotencia
- infertilidad
- bajo apetito sexual
- cansancio
- estados de ánimo depresivos
- pérdida ósea causada por un nivel hormonal bajo
2. Qué necesita saber antes de que le administren Reandron
No use Reandron
- si es alérgico a la testosterona undecanoato o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si padece cáncer andrógeno dependiente o sospecha que puede padecer cáncer de próstata o de las mamas.
- si tiene o ha tenido un tumor del hígado;
Reandron no está indicadopara su uso en mujeres.
Advertencias y precauciones
Consulte a su médicoantes de empezar a usar Reandron si tiene o alguna vez ha tenido:
- epilepsia
- problemas de corazón, hígado o riñón
- migraña
- interrupciones de la respiración durante el sueño (apnea) ya que puede empeorar con el tratamiento
- cáncer, ya que deberá determinarse los niveles de calcio en sangre
- presión arterial alta o si está tratado de hipertensión arterial, ya que la testosterona puede causar un aumento de la presión arterial.
- problemas de coagulación
- trastornos hemorrágicos (por ejemplo hemofilia)
- trombofilia (anomalía de la coagulación de la sangre que aumenta el riesgo de trombosis - coágulos de sangre en los vasos sanguíneos)
- factores que aumentan el riesgo de coágulos sanguíneos en una vena: coágulos sanguíneos previos en una vena, fumar, obesidad, cáncer, inactividad, si un familiar directo ha tenido un coágulo de sangre en una pierna, pulmón u otro órgano a una temprana edad (p. ej., aproximadamente por debajo de los 50 años de edad), o a medida que envejece.
Cómo reconocer un coágulo de sangre: hinchazón dolorosa de una pierna o cambio repentino en el color de la piel, por ejemplo, volverse pálido, rojo o azul, dificultad para respirar repentina, tos repentina e inexplicable que puede provocar sangre, o dolor repentino en el pecho, mareos o vértigos severos, dolor intenso en el estómago, pérdida repentina de la visión. Busque atención médica urgente si experimenta alguno de estos síntomas.
Si padece insuficiencia grave del corazón, hígado o riñón,el tratamiento con Reandron puede causarle complicaciones graves en forma de retención de líquidos, que se puede acompañar en ocasiones de insuficiencia cardíaca (congestiva).
Antes de iniciar el tratamiento y durante el mismo, su médico comprobará los siguientes parámetros en su análisis de sangre: nivel de testosterona y hemograma completo.
Si su hígado no funciona bien
No se han realizado estudios formales en pacientes con insuficiencia hepática. Si alguna vez ha tenido un tumor en el hígado, no le recetarán Reandron (ver “No utilice Reandron”).
Niños y adolescentes
Reandron noestá indicadopara su uso en niños y adolescentes. No existe información disponible sobre el uso de Reandron en varones menores de 18 años.
Pacientes de edad avanzada (65 años o más)
No es necesario ajustar la dosis si tiene más de 65 años. (Ver “Examen médico/Seguimiento”).
Musculación y pruebas de dopaje
Reandron noestá indicado para aumentar la musculatura en individuos sanos o para incrementar la resistencia física.
Reandron puede dar resultados positivos en el test de dopaje.
Abuso de sustancias y dependencia
Utilice siempre este medicamento exactamente como su médico o farmacéutico le han indicado.
El abuso de testosterona, especialmente si usted toma demasiada cantidad ya sea de este medicamento solo o bien conjuntamente con otros esteroides anabólicos androgénicos, puede causar graves problemas de salud a su corazón y vasos sanguíneos (que le pueden provocar la muerte), a su salud mental y/o al hígado.
Las personas que han abusado de la testosterona pueden volverse dependientes y pueden experimentar síntomas de abstinencia cuando la dosis cambia de manera significativa o cuando se interrumpe repentinamente su uso. No debe abusar de este medicamento porque le puede ocasionar graves problemas de salud, tanto si lo utiliza solo como en combinación con otros esteroides anabólicos androgénicos. (ver “Posibles efectos adversos”)
Examen médico / Seguimiento
Las hormonas masculinas pueden aumentar el crecimiento del cáncer de próstata o aumentar el tamaño de la glándula prostática (hipertrofia prostática benigna). Antes de iniciar el tratamiento con Reandron, su médico debe realizar un examen médico, con el fin de excluir el riesgo de un cáncer de próstata ya existente.
Su médico debe realizar un seguimiento periódico cuidadoso de la próstata y las mamas, especialmente en pacientes de edad avanzada. Su médico también le realizará periódicamente algunos análisis de sangre.
Se ha comunicado la aparición de tumores del hígado benignos (no cancerosos) y malignos (cancerosos) en pacientes que han sido tratados con productos hormonales como compuestos androgénicos.
Uso de Reandron con otros medicamentos
Informe a su médico o farmacéuticosi está utilizando o ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento, incluso los adquiridos sin receta médica. Su médico puede tener que ajustar la dosis si está tomando otros medicamentos, como por ejemplo:
- La hormona adrenocorticotrópica (ACTH) o corticosteroides (usados para tratar diversas enfermedades tales como reumatismo, artritis, reacciones alérgicas y asma). Reandron puede aumentar el riesgo de retención de líquidos, especialmente si tiene problemas de corazón o de hígado.
- Medicamentos que hacen que la sangre sea más fluida (anticoagulantes orales, derivados cumarínicos) ya que pueden aumentar el riesgo de sangrado. Su médico comprobará su dosis.
- Medicamentos para el tratamiento de la diabetes. Puede ser necesario ajustar la dosis de su medicamento antidiabético. Como otros andrógenos, la testosterona puede aumentar el efecto de la insulina.
Informe a su médico si sufre alguna alteración de la coagulación,ya que es importante que su médico conozca esta información para decidir si puede ser tratado con Reandron.
Reandron puede afectar también a los resultados de pruebas de laboratorio (por ejemplo de la glándula tiroides). Informe al médico o al personal del laboratorio de que está en tratamiento con Reandron.
Embarazo y lactancia
Reandron no está indicado para su uso en mujeres y no debe ser usado en mujeres embarazadas o en periodo de lactancia.
Fertilidad
El tratamiento con altas dosis de medicamentos con testosterona pueden interrumpir o reducir la producción de esperma de forma reversible (ver también “Posibles efectos adversos”).
Conducción y uso de máquinas
No se ha observado ningún efecto sobre la capacidad para conducir y utilizar máquinas.
Reandron contiene benzoato de bencilo
Este medicamento contiene 2000 mg de benzoato de bencilo en cada 4 ml ampolla/vial equivalente a 500 mg/ml.
3. Cómo usar Reandron
Su médico le inyectará Reandron (1 ampolla/vial) por vía intramuscular, muy lentamente. El tratamiento se repetirá a las 10-14 semanas, tiempo suficiente para mantener los niveles de testosterona sin que ésta se acumule en la sangre.
Reandron debe administrarse solamente por vía intramuscular. Se debe tener especial cuidado para no inyectar el producto en un vaso sanguíneo (ver “Administración”).
Comienzo del tratamiento
Antes de comenzar el tratamiento y durante la primera parte del mismo, su médico determinará sus niveles de testosterona en sangre. Su médico podrá administrarle una segunda inyección, como muy pronto, pasadas 6 semanas desde la primera inyección, con el fin de alcanzar rápidamente el nivel necesario de testosterona. Esto dependerá de sus síntomas y de sus niveles de testosterona.
Mantenimiento de los niveles de Reandron durante el tratamiento
El intervalo entre inyecciones debe mantenerse dentro del límite recomendado de 10 a 14 semanas. Su médico medirá sus niveles de testosterona de manera regular al finalizar un intervalo de inyecciones para asegurar que los niveles son correctos. Si los niveles son muy bajos, su médico podrá aumentar la frecuencia de las inyecciones. Si los niveles son muy altos, su médico podrá disminuir la frecuencia de las inyecciones. Recuerde sus días de tratamiento, ya que de lo contrario sus niveles de testosterona no estarán correctamente controlados.
Si piensa que el efecto de Reandron es demasiado intenso o muy débil, informe a su médico.
Si usa más Reandron del que debe
Los síntomas de haber recibido demasiado Reandron incluyen:
- Irritabilidad
- Nerviosismo
- Aumento de peso
- Erecciones frecuentes o de larga duración
Informe a su médico si tiene alguno de los síntomas anteriores. Su médico reducirá la frecuencia de las inyecciones o suspenderá el tratamiento.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Las reacciones adversas más frecuentesson acné y dolor en el lugar de aplicación de la inyección.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 personas)
- niveles anormalmente elevados de glóbulos rojos
- aumento de peso
- sofocos
- acné
- agrandamiento de la próstata y problemas asociados
- reacciones diversas en el lugar de administración de la inyección, como por ejemplo, dolor, hematoma o irritación
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- reacción alérgica
- aumento del apetito, alteraciones en algunos resultados del análisis de sangre, como por ejemplo, aumento del azúcar o de las grasas
- depresión, alteraciones emocionales, insomnio, inquietud, agresividad o irritabilidad
- dolor de cabeza, migraña o temblores
- alteraciones cardiovasculares, presión arterial elevada o mareos
- bronquitis, sinusitis, tos, respiración entrecortada, ronquidos o alteraciones de la voz
- diarrea o náusea
- alteraciones en los resultados de las pruebas hepáticas
- pérdida de cabello o diversas reacciones cutáneas (por ejemplo picor, enrojecimiento o piel seca)
- dolor en las articulaciones o en las extremidades, problemas musculares (por ejemplo espasmos, dolor o rigidez) o creatin fosfoquinasa aumentada en sangre
- alteraciones del conducto urinario (por ejemplo, disminución del flujo de orina, retención de orina, necesidad urgente de orinar durante la noche)
- alteraciones de la próstata (por ejemplo, displasia prostática, endurecimiento o inflamación de la próstata), alteraciones del apetito sexual, dolor de testículos, dolor, endurecimiento o agrandamiento de las mamas o aumento del nivel de hormonas masculinas y femeninas
- cansancio, debilidad generalizada, sudoración excesiva o sudoración nocturna
Efectos adversos raros(pueden afectar hasta 1 de cada 1.000 pacientes)
- El líquido oleoso de Reandron puede alcanzar los pulmones (microembolia pulmonar de las soluciones oleosas) y en raras ocasiones puede provocar signos y síntomas tales como tos, respiración entrecortada, malestar general, sudoración excesiva, dolor de pecho, mareos, pinchazos o desvanecimiento. Estas reacciones pueden ocurrir durante o inmediatamente después de la inyección y son reversibles.
Se han notificado sospechas de reacciones anafilácticas tras la inyección de Reandron.
Además de los referidos anteriormente, se han observado los siguientes efectos adversos tras el tratamiento con productos que contienen testosterona: nerviosismo, hostilidad, breves interrupciones de la respiración durante el sueño (apnea), reacciones en la piel como por ejemplo, caspa y seborrea, crecimiento excesivo del vello, erecciones más frecuentes y en casos muy raros color amarillento en la piel y los ojos (ictericia).
El tratamiento con dosis elevadas de testosterona generalmente interrumpe o reduce la producción de espermatozoides aunque vuelve a la normalidad tras interrumpir el tratamiento. El tratamiento de sustitución de testosterona en caso de testículos de bajo funcionamiento (hipogonadismo), puede provocar en raros casos erecciones persistentes y dolorosas (priapismo). Los tratamientos de larga duración o con dosis altas de testosterona pueden producir ocasionalmente un aumento de la retención de líquidos y edema (hinchazón debido a la retención).
En general, con los medicamentos a base de testosterona se ha observado un riesgo frecuente de aumento del recuento de glóbulos rojos, del hematocrito (porcentaje de glóbulos rojos en la sangre) y de la hemoglobina (el componente de los glóbulos rojos que transporta el oxígeno), identificados mediante análisis de sangre periódicos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Reandron
Mantener este medicamento fuera de la vista y del alcance de los niños.
Este medicamento no requiere condiciones especiales de conservación.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de su farmacia habitual. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Reandron
El principio activo es undecanoato de testosterona 250 mg/ml (correspondiente a 157,9 mg de testosterona). 1 ampolla/vial contiene 1.000 mg de undecanoato de testosterona (que corresponden a 631,5 mg de testosterona).
Los demás componentes son: benzoato de bencilo y aceite de ricino refinado.
Aspecto del producto y contenido del envase
Reandron es una solución oleosa clara, entre incolora y marrón amarillenta. Se presenta en ampollas de vidrio de color topacio/viales de vidrio de color topacio, que contienen 4 ml de solución inyectable.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización Grünenthal Pharma, S.A. Doctor Zamenhof, 36 28027 Madrid España | Responsable de la fabricación Bayer AG 13342 Berlín Alemania o EVER Pharma Jena GmbH Brüsseler Strasse 18 07747 Jena Alemania o Grünenthal GmbH Zieglerstrasse 6 52078 Aachen Alemania o Grünenthal Pharmaceuticals GmbH & Co. KG Philipp-Ott-Straße 3 51373 Leverkusen Alemania |
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
- Chipre, República Checa, Grecia, Dinamarca, Luxemburgo, Malta, Polonia y Portugal: Nebido
- Austria: Nebido 1000 mg/4 ml Injektionslösung
- Bélgica: Nebido 1000 mg/4 ml, oplossing voor injectie
- Bulgaria: ?????? 250 mg/ml ??????????? ???????
- Croacia: Nebido 1000 mg/4 ml otopina za injekcij
- Finlandia: Nebido 1000 mg/4 ml injektioneste, liuos
- Francia: Nebido 1000 mg/4 ml, solution injectable
- Alemania: Nebido 1000 mg Injektionslösung
- Hungría:Nebido 250 mg/mloldatos injekció
- Islandia: Nebido1000 mg/4 ml stungulyf, lausn
- Italia:NEBIDO 1000 mg/4ml soluzione iniettabile
- Lituania: Nebido1000mg/4ml injekcinis tirpalas
- Holanda: Nebido 1000 mg/4 ml
- Noruega:Nebido1000 mg/4 ml injeksjonsvæske, oppløsning
- Rumania: Nebido 1000 mg/ 4 ml solutie injectabila
- Eslovaquia: Nebido 1000 mg/4 ml injekcný roztok
- Eslovenia: Nebido 1000 mg/4 ml raztopina za injiciranje
- España: Reandron1000mg/4ml solución inyectable
- Suecia: Nebido, 1000 mg/4 ml injektionsvätska, lösning
- Reino Unido e Irlanda: Nebido 1000mg/4ml, solution for injection
Fecha de la última revisión de este prospecto:Octubre 2022
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
--------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
A temperaturas de almacenamiento en frío las propiedades de esta solución de base oleosa podrían cambiar temporalmente (por ejemplo, mayor viscosidad, turbidez). Si está almacenado a temperatura fría, el producto se debe llevar a temperatura ambiente o temperatura corporal antes de usarlo.
La solución para inyección intramuscular debe ser inspeccionada de forma visual antes de su uso, debiendo ser usadas solo aquellas soluciones claras y libres de partículas.
El contenido de una ampolla/vial debe inyectarse por vía intramuscular inmediatamente después de abrir la ampolla/vial.
El medicamento es para un único uso, debiendo desecharse el resto de solución no utilizada.
Administración
Debe prestarse mucho cuidado para no inyectar el medicamento en un vaso sanguíneo.
Como con todas las soluciones oleosas, Reandron debe inyectarse únicamente por vía intramuscular y muy lentamente. La microembolia pulmonar relacionada con las soluciones oleosas puede provocar en raras ocasiones signos y síntomas tales como tos, disnea, malestar, hiperhidrosis, dolor en el pecho, mareos, parestesia o síncope. Estas reacciones pueden ocurrir durante o inmediatamente después de la inyección y son reversibles. El tratamiento es normalmente de apoyo, por ejemplo, administración de oxígeno suplementario.
Se han comunicado sospechas de reacción anafiláctica tras la administración de Reandron.
Advertencias
Deberá realizarse un control regular cuidadoso de la glándula prostática y de las mamas según los métodos establecidos (examen rectal digital y determinación del PSA en suero) en los pacientes que reciben tratamiento con testosterona, al menos una vez al año y dos veces al año en los pacientes de edad avanzada y de riesgo (aquellos con factores clínicos o familiares).
Además de las pruebas de laboratorio de determinación de la concentración de testosterona en sangre en los pacientes en tratamiento de larga duración, deberán realizarse periódicamente las siguientes pruebas de laboratorio: hemoglobina, hematocrito, pruebas de la función hepática y perfil lipídico.
En pacientes con insuficiencia cardiaca, hepática o renal grave o con cardiopatía isquémica, el tratamiento con testosterona puede causar complicaciones graves, caracterizadas por edema con o sin insuficiencia cardiaca congestiva. En este caso, se debe interrumpir el tratamiento inmediatamente.
Normas para abrir la ampolla de Reandron con sistema de apertura de “Un punto de corte” (UPC)
La ampolla tiene una marca por debajo del punto coloreado. Antes de abrir, asegúrese de que no queda solución en la parte superior de la ampolla. Utilice ambas manos para abrirla; sujetar con una mano la parte inferior de la ampolla y utilizar la otra mano para presionar hacia fuera y romper la parte superior de la ampolla en dirección opuesta al punto coloreado.

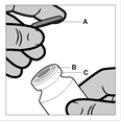
Normas para abrir el vial de Reandron
El vial es para un solo uso. El contenido del vial debe inyectarse por vía intramuscular inmediatamente después de cargarse a la jeringa. Tras retirar la cubierta de plástico (A) no retirar el anillo metálico (B) ni la cubierta del reborde (C).
- País de registro
- Precio medio en farmacia89.61 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a REANDRON 1000 mg/4 ml SOLUCION INYECTABLEForma farmacéutica: GEL, 2% testosteronaPrincipio activo: testosteronaFabricante: Advanz Pharma LimitedRequiere recetaForma farmacéutica: GEL, 23 mg/gPrincipio activo: testosteronaFabricante: The Simple Pharma Company LimitedRequiere recetaForma farmacéutica: INYECTABLE, TESTOSTERONA PROPIONATO 25 mg/mlPrincipio activo: testosteronaFabricante: Desma Laboratorio Farmaceutico S.L.Requiere receta
Médicos online para REANDRON 1000 mg/4 ml SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de REANDRON 1000 mg/4 ml SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes












