
MYCOSTATIN 100,000 IU/ml ORAL SUSPENSION


How to use MYCOSTATIN 100,000 IU/ml ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What MYCOSTATIN 100,000 IU/ml Oral Suspension is and what it is used for
- What you need to know before you take MYCOSTATIN 100,000 IU/ml Oral Suspension
- How to take MYCOSTATIN 100,000 IU/ml Oral Suspension
- Possible Side Effects
- Storage of MYCOSTATIN 100,000 IU/ml Oral Suspension
- Contents of the Pack and Other Information
Introduction
Package Leaflet: Information for the User
MYCOSTATIN 100,000 IU/ml Oral Suspension
Nystatin
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet:
- What MYCOSTATIN 100,000 IU/ml Oral Suspension is and what it is used for
- What you need to know before you take MYCOSTATIN 100,000 IU/ml Oral Suspension
- How to take MYCOSTATIN 100,000 IU/ml Oral Suspension
- Possible side effects
- Storage of MYCOSTATIN 100,000 IU/ml Oral Suspension
- Contents of the pack and other information
1. What MYCOSTATIN 100,000 IU/ml Oral Suspension is and what it is used for
MYCOSTATIN is an antifungal medicine used to treat oral and intestinal infections caused by a fungus called Candida.
2. What you need to know before you take MYCOSTATIN 100,000 IU/ml Oral Suspension
Do not take MYCOSTATIN
- if you are allergic to nystatin or any of the other ingredients of this medicine (listed in section 6).
- for the treatment of generalized infections of the body.
Warnings and Precautions
Consult your doctor or pharmacist before taking MYCOSTATIN.
In case of irritation or sensitization, consult your doctor immediately.
If you do not get a therapeutic response after 14 days of taking this medicine, consult your doctor.
Even if relief of symptoms occurs in the first few days of treatment, do not stop taking this medicine until the end of the treatment period indicated by your doctor.
Taking MYCOSTATIN with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
After taking MYCOSTATIN, do not take substances that modify intestinal movement or that can isolate the intestinal mucosa from nystatin, as the action of nystatin may be diminished.
Taking MYCOSTATIN with food and drinks
No interactions with food or drinks have been described; however, you should avoid taking substances that can affect intestinal movement or isolate the intestinal mucosa, and thus diminish the action of nystatin, after taking this medicine.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and Using Machines
MYCOSTATIN does not affect the ability to drive and use machines.
MYCOSTATIN containssucrose, ethanol, methylparaben (E-218), propylparaben (E-216), and sodium
This medicine contains 500 mg of sucrose per ml, which should be taken into account in patients with diabetes mellitus. If your doctor has told you that you have an intolerance to some sugars, consult with them before taking this medicine.
This medicine contains 455 mg of alcohol (ethanol) in each 60 ml, which corresponds to an amount of 0.76 g in each 100 ml. The amount in 1 ml of this medicine is equivalent to less than 0.2 ml of beer or 0.1 ml of wine.
The small amount of alcohol in this medicine does not produce any noticeable effect.
It may cause allergic reactions (possibly delayed), as it contains methylparaben (E-218) and propylparaben (E-216).
This medicine contains less than 1 mmol of sodium (23 mg) per ml, which is essentially "sodium-free".
3. How to take MYCOSTATIN 100,000 IU/ml Oral Suspension
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Your doctor will indicate the duration of your treatment with MYCOSTATIN. The treatment should continue for at least 48 hours after the disappearance of symptoms.
If the signs and symptoms worsen or persist after 14 days of treatment, the patient should be reevaluated and an alternative treatment should be considered.
The recommended dose is:
Adults:
- Oral candidiasis: 2.5 - 5 ml (250,000 - 500,000 IU) every 6-12 hours.
- Intestinal candidiasis: 5 - 10 ml (500,000 - 1,000,000 IU) every 6 hours.
Pediatric population:
- Oral candidiasis
- Infants over 1 year, children, and adolescents: 2.5 - 5 ml (250,000 - 500,000 IU) every 6-12 hours.
- Infants under or equal to 1 year: 2.5 ml (250,000 IU) every 6 hours.
- Newborns and infants with low birth weight: 1 ml (100,000 IU) every 6 hours.
- Intestinal candidiasis:
- Children and adolescents: 2.5 - 7.5 ml (250,000 - 750,000 IU) every 6 hours.
- Infants: 1 - 3 ml (100,000 - 300,000 IU) every 6 hours.
Method of administration:
A dosing syringe is included in the package for oral administration.
First use:
- Remove the adapter from the syringe (see image 1)
- Remove the cap from the vial
- Insert the adapter into the vial (see image 2)
- Cover the vial.
Each time you use it:
- Preparing the delivery device
- Shake the suspension before each use
- Remove the cap from the vial
- Place the syringe in the adapter (see image 3)
- Turn the vial/syringe assembly upside down (see image 4)
- Fill the syringe with a small amount of medicine by pulling the plunger and then pushing the plunger up to avoid the formation of air bubbles.
- Preparing the dose
- Make sure the plunger is at the bottom of the syringe (see image 5)
- Slowly pull the plunger to extract the required dose with the dosing syringe
- Once the dose is extracted, turn the vial/syringe assembly upside down and remove it by gently twisting the syringe containing the dose to be administered
- Insert the tip of the syringe directly into the mouth
- Administer the entire extracted volume immediately by slowly pressing the plunger.
After each use:
- Close the vial with the cap, leaving the adapter in the vial
- Rinse the syringe with water, repeating the action
- Store the syringe dry in the package.
Never separate the dosing syringe from the other components of the medicine package (box, leaflet).
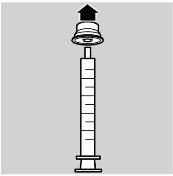
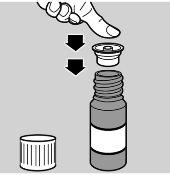
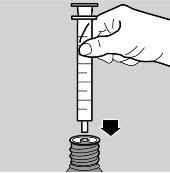
Image 1 Image 2 Image 3
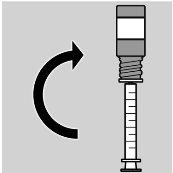
Image 4 Image 5
The suspension can be administered alone, with the help of water, or mixed with a liquid or soft food that is not acidic, such as milk, honey, jelly, etc.
In case of oral candidiasis, keep the suspension in the mouth for as long as possible (several minutes) before swallowing. For infants or small children, apply half the dose to each side of the mouth.
If you take more MYCOSTATIN than you should
Given the low absorption of this medicine, the possibility of intoxication, even by accidental ingestion, is very unlikely.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to take MYCOSTATIN
If you forget to take a dose, and if it is not close to the next dose, wait to take the next dose. Do not take a double dose to make up for forgotten doses.
If you stop taking MYCOSTATIN
Do not stop treatment before your doctor indicates, as resistance to the medicine may occur, making it difficult to treat subsequent reinfections.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed:
Uncommon (may affect up to 1 in 100 people): nausea, vomiting, gastrointestinal discomfort, and diarrhea, especially with high doses.
In some cases, the appearance of rash (skin eruption), including urticaria, has been reported. Very occasionally, Stevens-Johnson syndrome (characterized by skin, mucous membrane, and eye disorders) has been manifested. Cases of hypersensitivity (allergy) and angioedema (inflammation of the deeper layers of the skin), including facial edema (fluid accumulation in the face), have also been reported.
Reporting of Side Effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of MYCOSTATIN 100,000 IU/ml Oral Suspension
Keep this medicine out of the sight and reach of children.
Before opening the bottle for the first time, store below 30°C.
After opening the bottle, store below 25°C; in these conditions, the expiration period is 7 days.
Do not use this medicine after the expiration date stated on the package after EXP. The expiration date is the last day of the month indicated.
Do not use this medicine if you notice any visible signs of deterioration (e.g., change in appearance of the suspension or its color, odor, or taste).
Medicines should not be disposed of via wastewater or household waste. Place the package and any unused medicine in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the package and any unused medicine. This will help protect the environment.
6. Contents of the Pack and Other Information
Composition of MYCOSTATIN
- The active substance is nystatin. Each ml of oral suspension contains 100,000 IU of nystatin.
- The other ingredients are sucrose, ethanol 96%, sodium carmellose (E-466), cinnamaldehyde, peppermint flavor, cherry flavor, anhydrous disodium phosphate, glycerol (E-422), methylparaben (E-218), propylparaben (E-216), sodium hydroxide, hydrochloric acid, and purified water.
Appearance of the Product and Contents of the Pack
MYCOSTATIN is presented as a yellowish, opaque, and homogeneous oral suspension with a cherry and mint flavor.
Each package contains a bottle with 30 or 60 ml of suspension, accompanied by a graduated dosing syringe from 0.5 to 5 ml (for oral use) and an adapter to fix it to the bottle.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
SUBSTIPHARM
24 rue Erlanger
75016 Paris
France
Manufacturer:
Vetprom AD
The Vpharma site,
Otets Paisiy Str.
Radomir, 2400,
Bulgaria
Date of the Last Revision of this Leaflet: June 2025.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price4.68 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MYCOSTATIN 100,000 IU/ml ORAL SUSPENSIONDosage form: TABLET, 200 mg fidaxomicinActive substance: fidaxomicinManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 40 mg/mlActive substance: fidaxomicinManufacturer: Tillotts Pharma GmbhPrescription requiredDosage form: CAPSULE, 250 mgActive substance: paromomycinManufacturer: Pfizer S.L.Prescription required
Online doctors for MYCOSTATIN 100,000 IU/ml ORAL SUSPENSION
Discuss questions about MYCOSTATIN 100,000 IU/ml ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















