JEVTANA 60 MG CONCENTRADO Y DISOLVENTE PARA SOLUCION PARA PERFUSION

Cómo usar JEVTANA 60 MG CONCENTRADO Y DISOLVENTE PARA SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
JEVTANA 60 mg concentrado y disolvente para solución para perfusión
cabazitaxel
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermera.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermera, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es JEVTANA y para qué se utiliza
- Qué necesita saber antes de que le administren JEVTANA
- Cómo usar JEVTANA
- Posibles efectos adversos
- Conservación de JEVTANA
- Contenido del envase e información adicional
1. Qué es JEVTANA y para qué se utiliza
El nombre de su medicamento es JEVTANA. Su denominación común es cabazitaxel. Pertenece a un grupo de medicamentos denominado “taxanos”, utilizados para tratar cánceres.
JEVTANA se utiliza para el tratamiento del cáncer de próstata que ha progresado después de haber recibido otra quimioterapia. Actúa deteniendo el crecimiento de las células y su multiplicación.
Como parte de su tratamiento, tomará también cada día un corticosteroide (prednisona o prednisolona), por vía oral. Pida información a su médico sobre este otro medicamento.
2. Qué necesita saber antes de que le administren JEVTANA
No use JEVTANA
- si es alérgico (hipersensible) a cabazitaxel, a otros taxanos, al polisorbato 80 o a alguno de los demás componentes de este medicamento (incluidos en la sección 6),
- si el número de sus glóbulos blancos es muy bajo (número de neutrófilos menor o igual a 1.500/mm3),
- si tiene problemas graves de hígado,
- si recientemente ha sido o va a ser vacunado contra la fiebre amarilla.
No debe recibir JEVTANA si le sucede alguna de las circunstancias anteriores. Si no está seguro, consulte a su médico antes de recibir JEVTANA.
Advertencias y precauciones
Antes de iniciar el tratamiento con JEVTANA, le harán análisis de sangre para comprobar que tiene suficientes células sanguíneas y que sus riñones e hígado funcionan adecuadamente para recibir JEVTANA.
Informe a su médico inmediatamente si:
- tiene fiebre. Durante el tratamiento con JEVTANA es más probable que se reduzca el número de sus glóbulos blancos. El médico controlará su sangre y su estado general para detectar signos de infecciones. Podría administrarle otros medicamentos para mantener el número de sus células sanguíneas. Las personas con recuentos celulares bajos pueden desarrollar infecciones que pueden poner en peligro la vida. El primer signo de infección podría ser fiebre, por lo que si tiene fiebre, informe a su médico inmediatamente.
- alguna vez ha tenido cualquier alergia. Durante el tratamiento con JEVTANA pueden producirse reacciones alérgicas graves.
- tiene diarrea grave o duradera, se siente mal (náuseas) o está mal (vómitos). Cualquiera de estas situaciones puede producir deshidratación grave. Su médico tendría que ponerle un tratamiento.
- tiene sensación de insensibilidad, hormigueo, ardor o disminución de las sensaciones en manos y pies.
- tiene algún problema de sangrado en el intestino o tiene cambios en el color de sus heces o dolor de estómago. Si el sangrado o el dolor es grave, su médico interrumpirá su tratamiento con JEVTANA. Esto es porque JEVTANA podría incrementar el riesgo de sangrado o desarrollo de perforaciones en la pared intestinal.
- tiene problemas de riñón.
- tiene piel y ojos amarillentos, orina oscura, náuseas intensas (sensación de malestar) o vómitos, ya que pueden ser signos o síntomas de problemas hepáticos.
- nota que el volumen de su orina aumenta o disminuye significativamente.
- tiene sangre en su orina.
Si le sucede cualquiera de las circunstancias anteriores, informe a su médico inmediatamente. Su médico podría reducir la dosis de JEVTANA o interrumpir el tratamiento.
Uso de JEVTANA con otros medicamentos
Informe a su médico, farmacéutico o enfermera si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta. Esto se debe a que algunos medicamentos pueden afectar la eficacia de JEVTANA o JEVTANA puede afectar la eficacia de otros medicamentos. Estos medicamentos incluyen los siguientes:
- ketoconazol, rifampicina (para infecciones);
- carbamazepina, fenobarbital o fenitoína (para convulsiones);
- Hierba de San Juan o hipérico (Hypericum perforatum) (planta medicinal utilizada para tratar la depresión y otros problemas);
- estatinas (tales como simvastatina, lovastatina, atorvastatina, rosuvastatina, o pravastatina) (para reducir el colesterol en su sangre);
- valsartan (para la hipertensión);
- repaglinida (para la diabetes).
Mientras esté en tratamiento con JEVTANA, consulte con su médico antes de vacunarse.
Embarazo, lactancia y fertilidad
JEVTANA no está indicado para el uso en mujeres.
Use preservativos en sus relaciones sexuales si su pareja está o pudiera estar embarazada. JEVTANA podría estar presente en su semen y puede afectar al feto. Se recomienda no engendrar un hijo durante y hasta 4 meses después del tratamiento y solicitar información sobre la conservación del esperma antes del tratamiento, ya que JEVTANA podría alterar la fertilidad masculina.
Conducción y uso de máquinas
Durante el tratamiento con este medicamento podría sentirse cansado o mareado. Si esto sucede, no conduzca ni use herramientas o máquinas hasta que se sienta mejor.
JEVTANA contiene etanol (alcohol)
Este medicamento contiene 573 mg de alcohol (etanol) en cada vial de disolvente. La cantidad en la dosis de este medicamento es equivalente a menos de 11 ml de cerveza o 5 ml de vino. La pequeña cantidad de alcohol que contiene este medicamento no produce ningún efecto perceptible. Si tiene adicción al alcohol, tiene una enfermedad hepática o epilepsia, consulte con su médico o farmacéutico antes de tomar este medicamento.
3. Cómo usar JEVTANA
Instrucciones de uso
Antes de recibir JEVTANA le administrarán medicamentos antialérgicos para reducir el riesgo de reacciones alérgicas.
- JEVTANA será administrado por un médico o una enfermera.
- JEVTANA debe prepararse (diluirse) antes de administrarse. Con este prospecto se proporciona información práctica para la manipulación y administración de JEVTANA para médicos, enfermeras y farmacéuticos.
- JEVTANA se administrará en el hospital mediante un gotero (perfusión) en una de sus venas (vía intravenosa) durante aproximadamente 1 hora.
- Como parte de su tratamiento, tomará también un medicamento corticosteroide (prednisona o prednisolona) por vía oral todos los días.
Cuánto y con qué frecuencia se administra
- La dosis habitual depende de su área de superficie corporal. Su médico calculará su área de superficie corporal en metros cuadrados (m2) y decidirá la dosis que debe recibir.
- Habitualmente recibirá una perfusión cada 3 semanas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermera.
4. Posibles efectos adversos
Al igual que todos los medicamentos, JEVTANA puede producir efectos adversos, aunque no todas las personas los sufran. Su médico comentará esto con usted y le explicará los riesgos y los beneficios potenciales de su tratamiento.
Acuda inmediatamente al médico si nota cualquiera de los siguientes efectos adversos:
- fiebre (temperatura alta). Esto es frecuente (podría afectar hasta 1 de cada 10 personas).
- pérdida grave de fluidos corporales (deshidratación). Esto es frecuente (podría afectar hasta 1 de cada 10 personas). Esto puede ocurrir si tiene diarrea grave o duradera, o fiebre, o si ha estado vomitando.
- dolor de estómago grave o dolor de estómago que no se resuelve. Esto puede suceder si usted tiene una perforación en el estómago, esófago, intestino (perforación gastrointestinal). Esto puede causar la muerte.
Si le sucede alguna de las circunstancias anteriores, comuníquelo inmediatamente a su médico.
Otros efectos adversos incluyen:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
- reducción del número de células sanguíneas rojas (anemia), o blancas (que son importantes para combatir las infecciones)
- reducción del número de plaquetas (lo cual resulta en un aumento del riesgo de tener hemorragias)
- pérdida de apetito (anorexia)
- molestias de estómago, incluyendo náuseas, vómitos, diarrea o estreñimiento
- dolor de espalda
- sangre en la orina
- cansancio, debilidad o falta de energía.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- alteración del gusto
- respiración entrecortada
- tos
- dolor abdominal
- pérdida de cabello a corto plazo (en la mayoría de los casos el pelo vuelve a crecer con normalidad)
- dolor de las articulaciones
- infección del tracto urinario
- escasez de glóbulos blancos asociada con fiebre e infecciones
- sensación de insensibilidad, hormigueo, ardor o disminución de las sensaciones en manos y pies
- mareo
- dolor de cabeza
- aumento o disminución de la tensión arterial
- malestar de estómago, ardor de estómago o eructos
- dolor de estómago
- hemorroides
- espasmos musculares
- orinar con frecuencia o con dolor
- incontinencia urinaria
- problemas o alteración de los riñones
- úlceras en la boca o en los labios
- infecciones o riesgo de infecciones
- nivel de azúcar en sangre elevado
- insomnio
- confusión mental
- sensación de ansiedad
- sensación rara o pérdida de sensación o dolor en manos y pies
- problemas de equilibrio
- latidos rápidos o irregulares del corazón
- coágulos de sangre en las piernas o en el pulmón
- sensación de sofoco en la piel
- dolor de boca o garganta
- hemorragia rectal
- molestias, trastornos, debilidad o dolores musculares
- inflamación de pies o piernas
- escalofríos.
- trastornos en las uñas (cambio de color en sus uñas; las uñas se podrían desprender).
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- nivel de potasio en sangre bajo
- zumbidos en los oídos
- sensación de calor en la piel
- piel enrojecida
- inflamación de la vejiga, que puede ocurrir cuando su vejiga ha estado previamente expuesta a radioterapia (cistitis debida a fenómenos de recuerdo de radiación).
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- enfermedad pulmonar intersticial (inflamación de los pulmones causando tos y dificultad para respirar).
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de JEVTANA
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y en la etiqueta de los viales después de CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 30ºC. No refrigerar.
En la sección “INFORMACIÓN PRÁCTICA PARA MÉDICOS O PROFESIONALES SANITARIOS SOBRE LA PREPARACIÓN, ADMINISTRACIÓN Y MANIPULACIÓN DE JEVTANA” se incluye información sobre la conservación y el tiempo de uso de JEVTANA, una vez que se ha diluido y está listo para usar.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local. Estas medidas ayudarán a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de JEVTANA
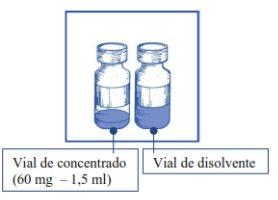
El principio activo es cabazitaxel. Un ml de concentrado contiene 40 mg de cabazitaxel. Un vial de concentrado contiene 60 mg de cabazitaxel.
Los demás componentes son polisorbato 80 y ácido cítrico en el concentrado, y etanol 96% y agua para preparaciones inyectables en el disolvente (ver sección 2 “JEVTANA contiene etanol (alcohol)”).
Nota: tanto el vial del concentrado de JEVTANA 60 mg/1,5 ml (volumen de llenado: 73,2 mg de cabazitaxel/1,83 ml) como el del vial de disolvente (volumen de llenado: 5,67 ml) contienen un sobrellenado para compensar la pérdida de líquido durante la preparación. Este sobrellenado asegura que después de la dilución con el contenido COMPLETOdel disolvente proporcionado, haya una solución conteniendo 10 mg/ml de cabazitaxel.
Aspecto del producto y contenido del envase
JEVTANA es un concentrado y disolvente para solución para perfusión (concentrado estéril).
El concentrado es una solución oleosa transparente, de color amarillo a amarillento-marronáceo.
El disolvente es una solución transparente e incolora.
Un envase de JEVTANA contiene:
- Un vial de un solo uso de vidrio transparente, cerrado con un tapón de caucho de color gris, sellado con una cápsula de aluminio, cubierta con un tapón expulsor flip-off de plástico de color verde claro, conteniendo 1,5 ml (volumen nominal) de concentrado.
- Un vial de un solo uso de vidrio transparente, cerrado con un tapón de caucho de color gris, sellado con una cápsula de aluminio dorada, cubierta con un tapón expulsor flip-off de plástico incoloro, conteniendo 4,5 ml (volumen nominal) de disolvente.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly
Francia
Responsable de la fabricación
Sanofi-Aventis Deutschland GmbH
Industriepark Höchst
65926 Frankfurt am Main
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Sanofi Belgium Tél.: +32 (0)2 710 54 00 | Luxembourg/Luxemburg Sanofi Belgium Tél.: +32 (0)2 710 54 00 (Bélgica) |
| Magyarország sanofi-aventis zrt Tél.: +36 1 505 0050 |
Ceská republika Sanofi s.r.o. Tél.: +420 233 086 111 | Malta Sanofi S.r.l. Tel: +39 02 39394275 |
Danmark Sanofi A/S Tél.: +45 45 16 70 00 | Nederland Sanofi B.V. Tél.: +31 20 245 4000 |
Deutschland sanofi-aventis Deutschland GmbH Tél.: 0800 04 36 996 Tel. aus dem Ausland: +49 69 305 70 13 | Norge sanofi-aventis Norge AS Tél.: +47 67 10 71 00 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Österreich sanofi-aventis GmbH Tél.: +43 1 80 185 – 0 |
Ελλ?δα Sanofi-Aventis Μονοπρ?σωπη AEBE Tél.: +30 210 900 16 00 | Polska Sanofi Sp. z.o.o. Tél.: +48 22 280 00 00 |
España sanofi-aventis, S.A. Tél.: +34 93 485 94 00 | Portugal Sanofi – Productos Farmacêuticos, Lda. Tél.: +351 21 35 89 400 |
France Sanofi Winthrop Industrie Tél.: 0 800 222 555 Llamar después al extranjero: +33 1 57 63 23 23 | România Sanofi Romania SRL Tél.: +40 (0)21 317 31 36 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tél.: +353 (0) 1 403 56 00 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ísland Vistor hf. Tél.: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tél.: +39.800.536389 | Suomi/Finland Sanofi Oy Tél.: +358 (0) 201 200 300 |
Κ?προς C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sverige Sanofi AB Tél.: +357 22 871600 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50 | United Kingdom (Northern Ireland) sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44 (0) 800 035 2525 |
Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www. ema.europa.eu.
–––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––––
La siguiente información está destinada únicamente a profesionales sanitarios.
INFORMACIÓN PRÁCTICA PARA MÉDICOS O PROFESIONALES SANITARIOS SOBRE LA PREPARACIÓN, ADMINISTRACIÓN Y MANIPULACIÓN DE JEVTANA 60 mg CONCENTRADO Y DISOLVENTE PARA SOLUCIÓN PARA PERFUSIÓN
Esta información complementa las secciones 3 y 5 para el usuario.
Es importante que lea el contenido completo de este procedimiento antes de preparar la solución para perfusión.
Incompatibilidades
Este medicamento no debe mezclarse con otros medicamentos excepto los utilizados para las diluciones.
Periodo de validez y precauciones especiales de conservación
Para el envase de JEVTANA 60 mg concentrado y disolvente
No conservar a temperatura superior a 30ºC.
No refrigerar.
Después de la apertura del vial
Los viales de concentrado y disolvente deben utilizarse inmediatamente. Si no se utilizan inmediatamente, el tiempo y las condiciones de conservación son responsabilidad del usuario. Desde un punto de vista microbiológico, el proceso de dilución en dos etapas debe realizarse en condiciones controladas y asépticas (ver a continuación “Precauciones de preparación y administración”).
Después de la dilución inicialde JEVTANA 60 mg concentrado con el contenido completodel vial de disolvente:
se ha demostrado la estabilidad química y física en uso durante 1 hora a temperatura ambiente.
Después de la dilución final en la bolsa/botella de perfusión
Se ha demostrado la estabilidad química y física de la solución de perfusión durante 8 horas a temperatura ambiente (15ºC - 30ºC) incluyendo 1 hora de tiempo de perfusión y durante 48 horas en nevera incluyendo la hora de tiempo de perfusión.
Desde un punto de vista microbiológico, la solución de perfusión debe utilizarse inmediatamente. Si no se utiliza inmediatamente, los tiempos y las condiciones de conservación son responsabilidad del usuario y normalmente no deberían ser más de 24 horas a 2ºC - 8ºC, a menos que la dilución se haya realizado en condiciones asépticas controladas y validadas.
Precauciones de preparación y administración
Al igual que otros agentes antineoplásicos, debe actuarse con precaución durante la preparación y administración de las soluciones de JEVTANA, teniendo en cuenta el uso de dispositivos de seguridad, equipo de protección personal (por ej. guantes) y procedimientos de preparación.
Si en cualquiera de las etapas de preparación, JEVTANA entrara en contacto con la piel, lavar inmediatamente y minuciosamente con agua y jabón. Si entrara en contacto con membranas mucosas, lavar inmediata y minuciosamente con agua.
JEVTANA sólo debe ser preparado y administrado por personal entrenado en el manejo de agentes citotóxicos. Las trabajadoras embarazadas no deben manipularlo.
Diluir siempre el concentrado para solución para perfusión con el disolvente completoque se proporciona antes de añadirlo a las soluciones de perfusión.
Etapas de la preparación
Lea detenidamente TODAesta sección antes de mezclar y diluir. JEVTANA requiere DOSdiluciones antes de la administración. Siga las instrucciones de preparación que se proporcionan a continuación.
Nota: tanto el vial del concentrado de JEVTANA 60 mg/1,5 ml (volumen de llenado: 73,2 mg de cabazitaxel/1,83 ml) como el vial de disolvente (volumen de llenado: 5,67 ml) contienen un sobrellenado para compensar la pérdida de líquido durante la preparación. Este sobrellenado asegura que después de la dilución con el contenido COMPLETOdel disolvente proporcionado, haya una solución conteniendo 10 mg/ml de cabazitaxel.
Para preparar la solución para perfusión, el siguiente proceso de dilución en dos etapas debe realizarse de forma aséptica.
Etapa1: dilución inicial del concentrado de solución para perfusión con el disolvente proporcionado.
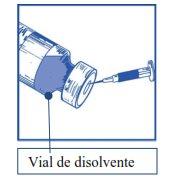
Etapa1.1 Inspeccionar el vial de concentrado y el disolvente proporcionado. La solución de concentrado y de disolvente deben ser transparentes. |
|
Etapa1.2 Utilizando una jeringa provista de una aguja fija, extraer de forma aséptica el contenido completodel disolvente proporcionado invirtiendo parcialmente el vial. |
|
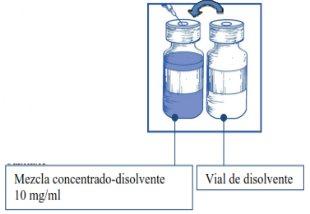
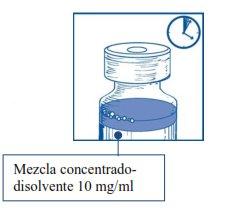
Etapa1.3 Inyectar el contenido completoen el correspondiente vial de concentrado. Para limitar todo lo posible la formación de espuma al inyectar el disolvente, dirigir la aguja hacia la pared interior del vial de solución de concentrado e inyectar lentamente. Una vez reconstituido, la solución resultante contiene 10 mg/ml de cabazitaxel. |
|
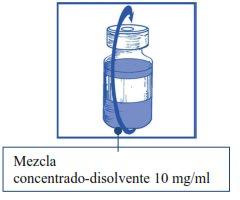
Etapa 1.4 Sacar la jeringa y la aguja y mezclar manualmente y suavemente, mediante inversiones repetidas, hasta que se obtenga una solución transparente y homogénea. Se pueden tardar unos 45 segundos. |
|
Etapa1.5 Dejar reposar la solución durante aproximadamente 5 minutos y a continuación comprobar que la solución es homogénea y transparente. Es normal que persista la espuma pasado este tiempo. |
|
Esta mezcla concentrado-disolvente resultante contiene 10 mg/ml de cabazitaxel (al menos 6 ml de volumen liberado). La segunda dilución se debe realizar inmediatamente (antes de 1 hora) como se detalla en la Etapa 2.
Podría ser necesario más de un vial de mezcla concentrado-disolvente para administrar la dosis prescrita.
Etapa2: segunda dilución (final) para perfusión
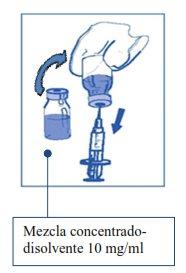
Etapa2.1 De forma aséptica extraer la cantidad requerida de mezcla concentrado-disolvente (10 mg/ml de cabazitaxel), con una jeringa graduada provista de una aguja fija. Como ejemplo, una dosis de 45 mg de JEVTANA requeriría 4,5 ml de la mezcla de concentrado-disolvente preparada en la Etapa 1. Como puede seguir habiendo espuma en la pared del vial de esta solución, después de la preparación descrita en la Etapa 1, es preferible situar la aguja de la jeringa en la mitad del contenido durante la extracción. |
|
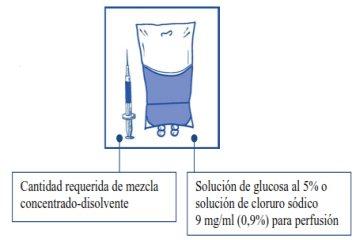
Etapa 2.2 Inyectar en un envase estéril sin PVC de solución de glucosa al 5% o solución de cloruro sódico 9 mg/ml (0,9%) para perfusión. La concentración de la solución para perfusión debe estar entre 0,10 mg/ml y 0,26 mg/ml. |
|
Etapa 2.3 Sacar la jeringa y mezclar el contenido de la bolsa o botella de perfusión manualmente, mediante movimiento de balanceo. |
|
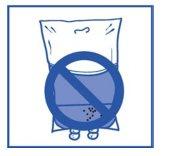
Etapa 2.4 Al igual que todos los productos parenterales, la solución de perfusión resultante debe inspeccionarse visualmente antes de usarla. Como la solución de perfusión está sobresaturada, puede cristalizar con el tiempo. En este caso, no se debe utilizar la solución y debe eliminarse. |
|
La solución para perfusión debe utilizarse inmediatamente. No obstante, el tiempo de conservación en uso puede ser más largo bajo las condiciones específicas mencionadas en la sección Periodo de validezy precauciones especiales de conservación.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él, se realizará de acuerdo con la normativa local.
Método de administración
JEVTANA se administra en perfusión durante 1 hora.
Se recomienda el uso de un filtro en línea de 0,22 micrómetros de tamaño de poro nominal (también denominado 0,2 micrómetros) durante la administración.
No deben utilizarse envases de perfusión de PVC o sets de perfusión de poliuretano para la preparación y administración de la solución para perfusión.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a JEVTANA 60 MG CONCENTRADO Y DISOLVENTE PARA SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 20 mg/mlPrincipio activo: CabazitaxelFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 20 MGPrincipio activo: CabazitaxelFabricante: Eugia Pharma (Malta) LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 60 mgPrincipio activo: CabazitaxelFabricante: Reddy Pharma Iberia S.A.Requiere receta
Médicos online para JEVTANA 60 MG CONCENTRADO Y DISOLVENTE PARA SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de JEVTANA 60 MG CONCENTRADO Y DISOLVENTE PARA SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes