
DAGAN 10 mg ORALLY DISPERSIBLE TABLETS

How to use DAGAN 10 mg ORALLY DISPERSIBLE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
DAGAN10mg Orodispersible Tablets EFG
Zolpidem Tartrate
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is DAGAN and what is it used for
- What you need to know before taking DAGAN
- How to take DAGAN
- Possible side effects
- Storage of DAGAN
- Contents of the pack and further information
1. What is DAGAN and what is it used for
Zolpidem tartrate is the active ingredient in DAGAN. This belongs to a group of medicines called hypnotics. It acts on the brain to help you sleep.
DAGAN is used to treat temporary sleep disorders in adults that are causing severe anxiety or affecting your daily life. These sleep disorders in adults include, among others:
- Difficulty falling asleep
Your doctor will identify your sleep problem and the factors that cause it, if possible, before prescribing this medication. If your sleep problems do not stop after treatment for 7 to 14 days, it may indicate that you have an underlying disorder, and your doctor will evaluate you periodically.
DAGAN is not intended for daily use over extended periods (treatment should be as short as possible and not exceed 4 weeks). If you are unsure, consult your doctor.
2. What you need to know before taking DAGAN
Do not takeDAGAN:
- if you are allergic to zolpidem tartrate or any of the other ingredients of this medication (listed in section 6). The signs of an allergic reaction are, among others: skin rash, swallowing or breathing problems, swelling of the lips, face, throat, or tongue.
- if your lungs do not function properly (respiratory insufficiency).
- if you have severe liver problems.
- if you have a condition where you stop breathing for short periods while sleeping (sleep apnea).
- if you have a disorder that causes severe muscle weakness (myasthenia gravis).
- if your doctor has told you that you have a mental illness (psychosis).
- if you are under 18 years of age.
Do not take this medication if any of the above applies to you. If you are unsure, consult your doctor or pharmacist before taking zolpidem.
Warnings and precautions
Consult your doctor or pharmacist before takingDAGANif:
- You have a history of alcohol or drug abuse.
- You have liver problems.
- You have depression or have had other mental illnesses in the past.
- You have recently taken zolpidem or other similar medications for more than four weeks.
- You have kidney problems.
- You have respiratory problems.
- You are elderly or weak.
Zolpidem may cause drowsiness and reduce your level of alertness. This could lead to falls and sometimes serious injuries.
If you are unsure whether the above applies to you, consult your doctor or pharmacist before taking zolpidem.
Duration of treatment
- Once you start taking DAGAN, you should know that it is for a limited time.
- You should take zolpidem for a short period, not exceeding 4 weeks.
- You should always consult your doctor as indicated to re-evaluate the situation.
Psychomotor impairment the next day (see also Driving and using machines)
The next day after taking zolpidem, the risk of psychomotor impairment, including impaired driving ability, increases if:
- You take this medication less than 8 hours before performing activities that require alertness.
- You take a higher dose than recommended.
- You take zolpidem with another central nervous system depressant or another medication that increases the effects of zolpidem in your blood, combined with alcohol or with illegal substances.
Take a single dose immediately before bedtime. Do not take another dose during the same night.
Other medications andDAGAN
Tell your doctor or pharmacist if you are taking, have recently taken, or may take any other medication. This is because zolpidem may affect how other medications work. Similarly, some medications may affect how zolpidem works.
Zolpidem may increase the effect of muscle relaxants.
Rifampicin, a medication used to treat tuberculosis, may decrease the effect of zolpidem.
Tell your doctor if you are taking any of the following medications:
DAGANmay increase the effect of the following medications:
If you take DAGAN with the following medications, it may increase drowsiness and the effects of psychomotor impairment the next day, including impaired driving ability:
- Medications for certain mental disorders (antipsychotics)
- Medications for sleep problems (hypnotics)
- Medications to relieve or reduce anxiety
- Medications for depression
- Medications for moderate to severe pain (narcotic analgesics)
- Medications for epilepsy
- Medications used for anesthesia
- Medications for seasonal allergic rhinitis, skin rashes, or other allergies that may cause drowsiness (sedating antihistamines)
If you take zolpidem with antidepressants, including bupropion, desipramine, fluoxetine, sertraline, and venlafaxine, you may see things that are not real (hallucinations).
If you take zolpidem with narcotic analgesics, you may feel euphoric and your dependence may increase.
It is not recommended to take zolpidem with fluvoxamine.
The following medications increase the likelihood of side effects when taken withDAGAN.To reduce this probability, your doctor may decide to reduce your dose of zolpidem:
- Certain antibiotics, such as ciprofloxacin (not recommended)
- Certain medications for treating fungal infections, such as ketoconazole or itraconazole
TakingDAGANwith food and drinks
- Do not drink alcoholwhile taking zolpidem. Alcohol may increase the effects of zolpidem and make you sleep so deeply that you may not breathe correctly or have difficulty waking up.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication. Taking DAGAN during pregnancy may harm your baby.
Zolpidem should not be taken during pregnancy, especially during the first three months. If, for urgent medical reasons, you take zolpidem during the last months of pregnancy or during childbirth, your baby may experience a drop in body temperature, muscle convulsions, and breathing difficulties, and may exhibit withdrawal symptoms after birth due to physical dependence.
Do not breastfeed if you are taking zolpidem, as this medication is excreted in small amounts in breast milk.
Consult your doctor or pharmacist before taking any medication if you are pregnant or breastfeeding.
Driving and using machines
DAGAN has a significant effect on the ability to drive and use machines, such as "driving while drowsy". The next day after taking zolpidem (as with other hypnotics), you should be aware that you may experience the following:
- You may feel drowsy, sleepy, dizzy, or confused
- You may take longer than usual to make decisions
- You may experience blurred or double vision
- Your level of alertness may be reduced
To reduce these effects, it is recommended to have a minimum period of 8 hours between taking zolpidem and driving, using machinery, or performing any work at heights.
Do not consume alcohol or any other psychoactive substance while taking zolpidem, as this may increase the aforementioned effects.
DAGANcontains:
- Lactose.If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
- Aspartame (E951):This medication contains 5 mg of aspartame in each tablet. Aspartame is a source of phenylalanine that may be harmful in cases of phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body is unable to eliminate it properly.
- Ammonio sulfite caramel (E150d):This medication may cause severe allergic reactions and bronchospasm (sudden feeling of suffocation) because it contains sulfites.
3. How to take DAGAN
Follow your doctor's instructions for taking DAGAN exactly. If you are unsure, consult your doctor or pharmacist again.
Taking this medication
- Take this medication by mouth.
- Place the tablet on your tongue and let it dissolve until it disintegrates without chewing. Swallow the resulting suspension with your saliva. You can then drink water or not.
- The recommended dose is 10 mg of DAGAN every 24 hours.
- A lower dose may be prescribed for some patients.
- Zolpidem should be taken as a single dose immediately before bedtime.
- Make sure you leave a minimum margin of 8 hours from taking the medication to performing activities that require alertness.
- Do not take more than 10 mg every 24 hours.
- The usual duration of treatment is 2 days to 4 weeks. The maximum treatment period, including the gradual dose reduction process, is four weeks.
Adults
The recommended dose is two 5 mg tablets or one 10 mg tablet (10 mg) just before bedtime. The maximum dose of 10 mg should not be exceeded.
Elderly patients (over 65 years) or weakened patients
The recommended dose is one 5 mg tablet just before bedtime. This recommended dose should not be exceeded.
Patients with liver problems
The initial dose is one 5 mg tablet just before bedtime. Your doctor will decide if the dose needs to be increased to two 5 mg tablets or one 10 mg tablet if they consider it safe to do so.
Do not take zolpidem if you have severe liver disorders.
Use in children and adolescents
Children under 18 years of age should not use DAGAN.
If you take moreDAGANthan you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 5620420, indicating the medication and the amount ingested.
Bring the medication packaging with you so that the doctor knows what you have taken. Taking too much zolpidem is very dangerous. The following effects may occur:
- drowsiness, mental confusion, hypotension, difficulty breathing, deep sleep, and possibility of falling into a coma.
If you forget to takeDAGAN
DAGAN should only be taken at bedtime. If you forget to take the dose at bedtime, do not take it at any other time, or you may feel drowsy, dizzy, and confused during the day.
Do not take a double dose to make up for missed doses.
If you forget to take the dose immediately before going to bed but remember during the night, simply take the missed dose if you can still sleep for 7 or 8 hours uninterrupted.
If you stop takingDAGAN
Continue taking DAGAN until your doctor tells you to stop. Do not stop taking zolpidem suddenly, but inform your doctor if you want to stop. Your doctor will need to reduce the dose and the tablets you take over a period of time.
If you stop taking zolpidem suddenly, you may have sleep problems again and experience what is known as "withdrawal symptoms". If this happens, you may notice the following effects:
Go to the doctor immediately if you experience any of the following effects:
- Feeling anxious and tense, restless, irritable, or confused
- Headache
- Nightmares, seeing or hearing things that are not real (hallucinations)
- Increased sensitivity to light, noise, and touch
- Alteration of the perception of reality
- Feeling distant from your body or like a puppet
- Numbness and tingling in hands and feet
- Muscle pain
- Worsening of sleep disorders
In rare cases, seizures (convulsions) may also occur.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Stop taking DAGANandinform your doctor or go immediately to a hospital if:
- You experience an allergic reaction. The signs of an allergic reaction are, among others: skin rash, difficulty swallowing or breathing, swelling of the lips, face, throat, or tongue. These adverse effects are serious but their frequency of occurrence is unknown (cannot be calculated from the available data). You need medical attention.
Inform your doctor as soon as possible if any of the following adverse effects appear:
Frequent(affect less than 1 in 10 people)
- Poor memory while taking zolpidem (amnesia) and strange behavior during this time, an effect that occurs with greater probability a few hours after taking the medicine. After 7-8 hours of sleep after taking zolpidem, it is less likely to cause problems.
- Difficulty sleeping that worsens after taking this medicine.
- Seeing or hearing things that are not real (hallucinations).
Infrequent(affect less than 1 in 100 people)
- Blurred vision or "double vision".
Unknown frequency(frequency cannot be estimated from the available data)
- Being less aware of your surroundings.
- Falls, especially in advanced ages.
Driving while drowsy and other unusual behaviors
Some cases have been reported of people doing things while asleep and not remembering them when they wake up, after taking a sleep medicine. These activities include driving while asleep, sleepwalking, or having sexual relations. Alcohol and some medications for depression or anxiety can increase the chances of these serious effects occurring.
Inform your doctor or pharmacist if any of the following side effects worsen or last more than a few days:
Frequent(affect less than 1 in 10 people)
- Diarrhea
- Feeling nauseous (nausea) or vomiting
- Abdominal pain
- Respiratory infection
- Headache
- Feeling tired or agitated
- Having nightmares
- Having a feeling of not being in contact with reality and being unable to think or judge clearly (psychosis)
- Feeling dizzy
- Feeling lethargic or drowsy
- Back pain
Infrequent(affect less than 1 in 100 people)
- Feeling confused or irritable
- Unusual sensations on the skin such as numbness, tingling, pinching, burning of the skin (paresthesia)
- Tremors
- Blurred vision
- Changes in appetite or appetite-related behavior
- Muscle pain
- Muscle spasms
Very rare(affect less than 1 in 10,000 people)
- Any alteration of vision, particularly loss of vision
Unknown frequency:(frequency cannot be estimated from the available data)
- Itching or skin rash
- Excessive sweating
- Slowing of breathing (respiratory depression)
- Muscle weakness
- Feeling restless, aggressive, angry, or showing unusual behavior
- Depression
- Exaggerated feeling of happiness/confidence (euphoria)
- Thinking things that are not real (delirium)
- Changes in sexual desire (libido)
- Alteration in the amount of liver enzymes (appears in blood test results)
- Change in skin or eye color, abdominal pain (stomach) or feeling of swelling, intense itching, light or bloody stools, extreme weakness, nausea, or loss of appetite. These signs may be due to an infection or liver injury.
- A disease in which the bile outlet from the liver is blocked (cholelithiasis).
- Signs such as jaundice (yellowing of the skin), skin rash, or fever, and darkening of urine color.
- Lack of concentration
- Speech problems
- Changes in gait
- Zolpidem has less effect than usual
- Becoming dependent on zolpidem
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of DAGAN
Keep this medicine out of sight and reach of children.
This medicine does not require special temperature conditions for its conservation. Keep it in the original packaging to protect it from light and moisture.
Do not use this medicine after the expiration date that appears on the box or blister after CAD. The expiration date is the last day of the month indicated.
Medicines should not be thrown down the drain or into the trash. Deposit the containers and medicines you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of DAGAN 10 mg oral dispersible tablets
- The active ingredient is zolpidem tartrate.
Each DAGAN tablet contains 10 mg of zolpidem tartrate.
- The other components (excipients) are potassium polyacrylate, mannitol, lactose monohydrate, microcrystalline cellulose, anhydrous colloidal silica, aspartame (E951), magnesium stearate, and blackcurrant flavoring (flavoring substances, corn maltodextrin (derived from corn starch), triethyl citrate, ammonium sulfite caramel (E150d)).
Appearance of the productand package contents
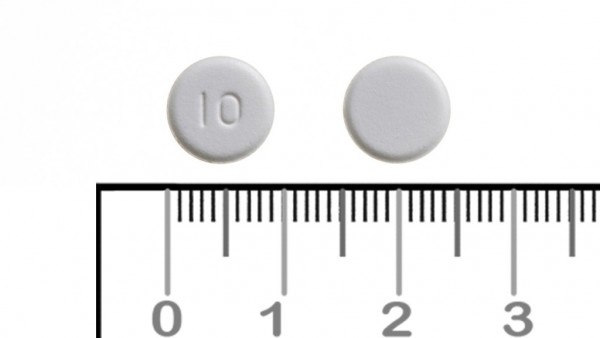
The 10 mg oral dispersible tablets are:
white to off-white, round, flat, and beveled, marked with "10" on one face and smooth on the other face, with a diameter of 10.0 mm ± 0.3 mm, thickness of 3.2 mm ± 0.3 mm, and a blackcurrant odor.
The oral dispersible tablets are packaged in an aluminum-aluminum blister
Components of the blister sheet - Base sheet: PVC-OPA-Aluminum-PVC; cover sheet: Aluminum-PVC
DAGAN 10 mg is available in packages of 28 and 30 oral dispersible tablets.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Meiji Pharma Spain, S.A.
Avda. de Madrid, 94
28802 Alcalá de Henares, Madrid (Spain)
Manufacturer:
LAMP SAN PROSPERO S.P.A
Via della Pace, 25/A
CP 41030 San Prospero
Módena
Italy
or
INPHARMASCI
ZI Nº 2 de Prouvy – Rouvignies
1 rue de Nungesser
59121 Prouvy
France
Date of the last revision of this leaflet: May 2025
Other sources of information:
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price2.78 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DAGAN 10 mg ORALLY DISPERSIBLE TABLETSDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 5 mgActive substance: zolpidemManufacturer: Meiji Pharma Spain S.A.Prescription requiredDosage form: TABLET, 10 mg zolpidem tartrateActive substance: zolpidemManufacturer: Sanofi Aventis S.A.Prescription requiredDosage form: TABLET, 10 mgActive substance: zolpidemManufacturer: Sanofi Aventis S.A.Prescription required
Online doctors for DAGAN 10 mg ORALLY DISPERSIBLE TABLETS
Discuss questions about DAGAN 10 mg ORALLY DISPERSIBLE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















