
ACARIZAX 12 SQ-HDM LIOFILIZADO SUBLINGUAL

Cómo usar ACARIZAX 12 SQ-HDM LIOFILIZADO SUBLINGUAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
ACARIZAX 12 SQ-HDM liofilizado sublingual
Para uso en adultos y niños (5-65 años de edad)
Extracto alergénico estandarizado de ácaros del polvo doméstico
(Dermatophagoides pteronyssinusy Dermatophagoides farinae)
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.Ver sección 4.
Contenido del prospecto
- Qué es ACARIZAX y para qué se utiliza
- Qué necesita saber antes de empezar a tomar ACARIZAX
- Cómo tomar ACARIZAX.
- Posibles efectos adversos
- Conservación de ACARIZAX
- Contenido del envase e información adicional
1. Qué es ACARIZAX y para qué se utiliza
ACARIZAX contiene un extracto alergénico de ácaros del polvo doméstico. Se presenta en una forma conocida como liofilizado sublingual, el cual es semejante a un comprimido, pero mucho más blando y se absorbe en el organismo al colocarlo debajo de la lengua.
ACARIZAX se usa para tratar la rinitis alérgica (inflamación de las membranas de la nariz) en adultos y niños (5-65 años de edad) y el asma alérgica asociada, causada por ácaros del polvo doméstico en adultos (18-65 años de edad). ACARIZAX actúa aumentando la tolerancia inmunológica (la capacidad de su cuerpo a actuar) frente a los ácaros del polvo doméstico. Puede ser necesario que tenga que tomar el tratamiento durante 8 a 14 semanas para que note alguna mejoría.
El médico comprobará sus síntomas alérgicos y le realizará una prueba cutánea de prick y/o le tomará una muestra de sangre para determinar si ACARIZAX es el tratamiento adecuado para usted.
Se debe tomar la primera dosis de ACARIZAX bajo supervisión médica. Debe permanecer en observación durante al menos media hora tras la toma de la primera dosis. Esto es una precaución que hay que tomar para observar su sensibilidad a este medicamento. Además le dará la posibilidad de comunicar a su médico cualquier posible efecto adverso que esté sufriendo.
ACARIZAX es recetado por médicos especialistas en el tratamiento de la alergia.
2. Qué necesita saber antes de empezar a tomar ACARIZAX
No tome ACARIZAX:
- si es alérgico a cualquiera de los excipientes (otros componentes) de este medicamento (incluidos en la sección 6).
- si su función pulmonar es pobre (diagnosticada por su médico).
- si ha sufrido un empeoramiento grave de su asma durante los últimos tres meses (diagnosticado por su médico).
- si el día que debe tomar la primera dosis de ACARIZAX padece asma y una infección en curso de las vías respiratorias, como un resfriado común, irritación de garganta o neumonía. Su médico retrasará el comienzo de su tratamiento hasta que se mejore.
- si padece una enfermedad que afecta a su sistema inmunitario; está tomando medicamentos que suprimen el sistema inmunitario o tiene cáncer.
- si se ha sometido recientemente a una extracción dental, o cualquier otra intervención en la boca, o tiene úlceras o infecciones en la boca. Su médico podría recomendarle retrasar el comienzo del tratamiento o interrumpir el tratamiento hasta que se le haya curado la boca.
Advertencias y precauciones
Consulte a su médico antes de empezar a tomar ACARIZAX:
- si está siendo tratado de depresión con antidepresivos tricíclicos, inhibidores de la monoamino oxidasa (IMAOs), o con inhibidores de la COMT para la enfermedad de Parkinson.
- si ha sufrido previamente una reacción alérgica grave a una inyección de un extracto alergénico de ácaros del polvo doméstico.
- si tiene alergia al pescado. ACARIZAX puede contener trazas de proteínas de pescado. Los datos disponibles no indican que haya un mayor riesgo de sufrir una reacción alérgica en pacientes alérgicos al pescado.
- si sufre síntomas alérgicos severos, como dificultad para tragar o respirar, cambios en la voz, hipotensión (presión arterial baja), o tiene sensación de tener un nudo en la garganta. Interrumpa el tratamiento y contacte con su médico inmediatamente.
- si sus síntomas asmáticos empeoran más de lo normal. Interrumpa el tratamiento y contacte con su médico inmediatamente.
Si padece asma, debe continuar usando su medicación habitual para el asma mientras comienza el tratamiento con ACARIZAX. Su médico le indicará como reducir su medicación para el asma de manera gradual a lo largo del tiempo.
Debe dejar de tomar ACARIZAX y ponerse en contacto con su médico si sufre ardor de estómago severo o persistente o dificultad al tragar, ya que éstos síntomas pueden ser signos de una inflamación alérgica del esófago.
Puede esperar sufrir alguna reacción alérgica local, de leve a moderada, durante su tratamiento. Sin embargo, si fuera grave, hable con su médico por si necesitara tomar alguna medicación antialérgica, como un antihistamínico.
Este medicamento contiene menos de 1 mmol de sodio (23mg) por dosis, esto es, esencialmente “exento de sodio”.
Niños
Rinitis alérgica (inflamación de las membranas de la nariz):
ACARIZAX no está destinado para su uso en niños menores de 5 años de edad.
Asma alérgica:
ACARIZAX no está destinado para el tratamiento del asma alérgica en niños menores de 18 años de edad.
Toma de ACARIZAX con otros medicamentos
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento, incluidos los medicamentos que se pueden adquirir sin receta. Si está tomando otros medicamentos para sus síntomas alergicos como antihistamínicos, medicamentos para aliviar el asma, o esteroides, dígaselo a su médico para que pueda aconsejarle acerca de su uso mientras se trata con ACARIZAX. Si deja de tomar estos medicamentos para sus síntomas alérgicos puede experimentar más efectos adversos a ACARIZAX.
Toma de ACARIZAX con alimentos y bebidas
No coma ni beba durante los 5 minutos siguientes a la toma de este medicamento.
Embarazo y lactancia
Actualmente no hay experiencia sobre el uso de ACARIZAX en el embarazo. No se debe iniciar el tratamiento con ACARIZAX durante el embarazo. Si se quedara embarazada durante el tratamiento, consulte con su médico si es apropiado para usted continuar con él.
Actualmente no hay experiencia sobre el uso de ACARIZAX durante la lactancia. Sin embargo, no se prevén efectos en el lactante. Consulte con su médico si puede continuar tomando ACARIZAX durante la lactancia.
Conducción y uso de máquinas
La influencia del tratamiento con ACARIZAX sobre la capacidad para conducir o utilizar máquinas es nula o despreciable.
Sin embargo, sólo usted puede juzgar si está afectando a sus capacidades. Por lo tanto, lea toda la información contenida en este prospecto, especialmente la sección 4 “Posibles efectos adversos” y en caso de duda, consulte a su médico o farmaceútico.
3. Cómo tomar ACARIZAX
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis habitual es de un liofilizado al día. Su médico le indicará durante cuanto tiempo debe tomar ACARIZAX.
Asegúrese de que sus manos están secas antes de tocar este medicamento. Tome el medicamento de la siguiente forma:
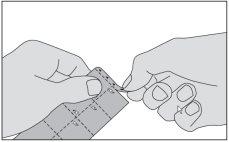
- Desprenda la tira marcada con triángulos en la parte superior del envase.
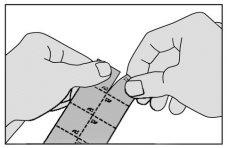
- Separe una unidad del envase siguiendo las líneas perforadas.
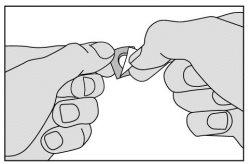
- No presione el medicamento para sacarlo a través del aluminio. Podría dañarlo, ya que se rompe con facilidad. En vez de eso, doble la esquina marcada de la lámina de aluminio y tire de ella.
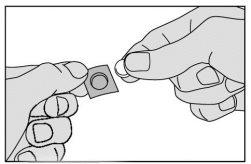
- Saque cuidadosamente el medicamento de la unidad y tómelo inmediatamente.
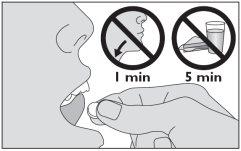
- Coloque el medicamento bajo la lengua. Deje que se disuelva ahí durante unos segundos. No trague durante el primer minuto. Espere por lo menos 5 minutos antes de comer o beber.
Si toma más ACARIZAX del que debe
Si ha tomado demasiados liofilizados puede presentar síntomas alérgicos, incluyendo síntomas locales en la boca y la garganta. Si experimenta síntomas graves, contacte inmediatamente con su médico u hospital.
Si olvidó tomar ACARIZAX
Si olvidó tomar una dosis, tómela más tarde en el día. No tome una dosis doble para compensar las dosis olvidadas.
Si olvidó tomar ACARIZAX durante más de 7 días, deberá contactar con su médico antes de volver a tomar ACARIZAX.
Si interrumpe el tratamiento con ACARIZAX
Si no toma este medicamento tal y como se lo han recetado, puede que no se beneficie de todos los efectos del tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmaceútico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos pueden ser una respuesta alérgica frente al alérgeno con el que se está tratando. La mayoría de los efectos adversos duran entre minutos y horas después de tomar el medicamento, y la mayoría remitirán cuando lleve 1-3 meses de tratamiento.
Los efectos adversos más graves:
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Reacción alérgica grave
Deje de tomar ACARIZAX y contacte inmediatamente con su médico u hospital si sufre algunos de los siguientes síntomas:
- Empeoramiento del asma existente
- Hinchazón de aparición rápida de la cara, boca o garganta
- Dificultad al tragar
- Dificultad al respirar
- Cambios en la voz
- Presión arterial baja
- Sensación de llenado en la garganta (como hinchada)
- Habones y picor de piel
Otros posibles efectos adversos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Sensación de irritación en la garganta
- Hinchazón en la boca y los labios
- Picor en la boca y los oídos
- Infección de las vías respiratorias
Frecuentes (pueden afectar hasta a 1 de cada 10 personas):
- Sensación de hormigueo o entumecimiento en la boca o la lengua
- Picor en los ojos
- Picor en la lengua o los labios
- Hinchazón en la lengua o garganta
- Inflamación, molestias o sensación de quemazón en la boca
- Enrojecimiento en la boca o úlceras en la boca
- Dolor en la boca
- Alteración del gusto
- Dolor de estómago o malestar
- Diarrea
- Malestar (náuseas) y vómitos
- Dolor o dificultad al tragar
- Síntomas de asma
- Tos
- Falta de aire
- Dolor en el pecho
- Indigestión y ardor de estómago
- Ronquera
- Cansancio
- Habones y picor en la piel
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Inflamación de los ojos
- Sensación de latidos cardiacos rapidos y fuertes o irregulares
- Molestia en el oído
Opresión en la garganta
- Molestias en la nariz, congestión o goteo nasal, estornudos
- Ampollas en la boca
- Irritación en el esófago
- Sensación de cuerpo extraño en la garganta
- Mareo
- Malestar general
- Boca seca
- Sensación de hormigueo en la piel
- Enrojecimiento de la garganta
- Aumento de tamaño de las amígdalas
- Dolor de labio
- Úlcera en el labio
- Agrandamiento de la glándula salival
- Aumento de la producción de saliva
- Enrojecimiento de la piel
Raras (pueden afectar hasta 1 de cada 1000 personas):
- Hinchazón de aparición rápida de la cara o la piel
- Inflamación alérgica del esófago (esofagitis eosinofílica)
Si alguno de los efectos adversos le preocupa o le está causando dificultades, debe contactar con su médico, el cual decidirá si necesita tomar algún medicamento, como los antihistamínicos, que le ayuden a aliviar los efectos adversos que está sufriendo.
Efectos adversos en niños
Los efectos adversos en niños se espera que sean similares a los efectos adversos en adultos y adolescentes.
Los efectos adversos que se puede esperar con más frecuencia en niños son:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas): hinchazón en la lengua o garganta, úlceras en la boca, dolor en la boca, alteración del gusto, dolor de estómago, diarrea, malestar (naúseas).
Frecuentes (pueden afectar hasta 1 de cada 10 personas): inflamación de los ojos
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas): rápida hinchazón de la cara y la piel e inflamación alérgica del esófago (esofagitis eosinofílica).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ACARIZAX
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice ACARIZAX después de la fecha de caducidad que aparece en el blíster y en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ACARIZAX
- El principio activo es un extracto alergénico estandarizado de ácaros del polvo doméstico, Dermatophagoides pteronyssinusy Dermatophagoides farinae. La actividad por liofilizado sublingual se expresa en unidades SQ-HDM. La actividad de un liofilizado sublingual es de 12 SQ-HDM.
- Los demás componentes son gelatina (procedente de pescado), manitol e hidróxido sódico (para ajuste de pH).
Aspecto del producto y contenido del envase
Liofilizado sublingual redondo, de color blanco o blanquecino, con un grabado en una de las caras.
Blísters de aluminio, con una de las láminas desplegable, envasados en una caja de cartón. Cada blíster contiene 10 liofilizados sublinguales.
Envases disponibles: 10, 30 y 90 liofilizados sublinguales.
Puede que no estén comercializados todos los tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
ALK-Abelló A/S
Bøge Allé 6-8
DK-2970 Hørsholm
Dinamarca.
Responsable de la fabricación
ALK-Abelló. S.A.
C/ Miguel Fleta, 19
28037 – Madrid
España
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
ALK-Abelló. S.A. C/ Miguel Fleta, 19 28037 – Madrid. | |
Este medicamento se encuentra autorizado en los Estados Miembros del Espacio Económico Europeo con los siguientes nombres:Bélgica, Croacia, Alemania, Hungría, Irlanda, Luxemburgo, Portugal, Rumanía, Eslovenia, España: ACARIZAX. Fecha de la última revisión de este prospecto:Diciembre 2024 La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/. |
- País de registro
- Precio medio en farmacia106.84 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ACARIZAX 12 SQ-HDM LIOFILIZADO SUBLINGUALForma farmacéutica: COMPRIMIDO SUBLINGUAL, 100 IR + 300 IRPrincipio activo: house dust mitesFabricante: StallergenesRequiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 100 mgPrincipio activo: house dust mitesFabricante: StallergenesRequiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 300 IRPrincipio activo: house dust mitesFabricante: StallergenesRequiere receta
Médicos online para ACARIZAX 12 SQ-HDM LIOFILIZADO SUBLINGUAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ACARIZAX 12 SQ-HDM LIOFILIZADO SUBLINGUAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















