
REGAINE 50 mg/g CUTANEOUS FOAM


How to use REGAINE 50 mg/g CUTANEOUS FOAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Regaine 50 mg/g Cutaneous Foam
Minoxidil
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
- You should consult a doctor if your condition worsens or does not improve after 4 months.
Contents of the Package Leaflet:
- What Regaine is and what it is used for
- What you need to know before starting to use Regaine
- How to use Regaine
- Possible side effects
- Storage of Regaine
- Contents of the pack and further information
1. What Regaine is and what it is used for
Regaine contains minoxidil. Minoxidil promotes hair regrowth and stabilizes hair loss in men with receding hairline and baldness on the crown, due to the revitalization of the hair follicle.
Regaine is used to treat hereditary hair loss (also known as androgenetic alopecia) in men. Use only on the scalp (head).
2. What you need to know before starting to use Regaine
Do not useRegaine
- if you are allergic to minoxidil or any of the other components of Regaine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Regaine if:
- you have any heart disease, including irregular heartbeat, angina, or chest pain and/or circulation disorders.
When minoxidil enters the bloodstream, it can cause side effects related to low blood pressure, such as chest pain, rapid heartbeat, weakness, dizziness, sudden and unexplained weight gain, or swollen hands and feet. It can also cause persistent redness or irritation of the scalp. If you experience any of these effects, stop treatment and consult your doctor.
Some patients have experienced changes in hair color and texture when using this medication.
A temporary increase in hair shedding (old hair falls out as new hair grows in its place) may occur during the first 2-6 weeks (small layers of scalp that come off) of treatment. This is a sign that Regaine is working, and new hair is replacing old hair. This effect usually disappears after two weeks. If hair shedding persists, you should stop using Regaine and consult your doctor.
Accidental contact
Accidental ingestion can cause serious cardiac side effects, so this product should be kept out of the reach of children.
If the foam is applied accidentally to other areas of the body besides the scalp, wash immediately with plenty of water to avoid unwanted hair growth.
There have been reports of excessive hair growth on the body of infants after skin contact with the application sites of minoxidil in patients (caregivers) using topical minoxidil. The hair growth returned to normal within a few months when the infants were no longer exposed to minoxidil. Care should be taken to ensure that children do not come into contact with areas of your body where you have applied minoxidil topically. Consult a doctor if you notice excessive hair growth on your child's body during the period you are using topical minoxidil products.
Do not use Regaine if:
- you have a different pattern of hair loss than receding hairline and baldness on the crown.
- you do not have a family history of hair loss.
- you have sudden and/or irregular hair loss.
- you do not know the reason for your hair loss.
- you have an inflamed, infected, irritated, or painful scalp.
Regaine 50 mg/g cutaneous foam is EXTREMELY FLAMMABLE!
Avoid contact with fire, flames, or smoke during and immediately after application.
Children and adolescents
Regaine should not be used by children and adolescents under 18 years of age.
Using Regaine with other medications
Tell your doctor or pharmacist if you are using, have recently used, or may use other medications.
Regaine should not be used in conjunction with other medications applied topically to the scalp.
Using Regaine with other hair care products
Using shampoo during treatment
You should use a mild shampoo in conjunction with Regaine. The scalp should be completely dry before applying Regaine.
Using beauty products for hair during treatment
For better results, Regaine should be applied to the scalp before using any beauty products. First, apply Regaine, and then apply your usual beauty products. Do not mix with other products for use on the scalp.
Advice for people with dyed or permed hair
There is no available information regarding a change in the effects of Regaine due to these treatments. However, to avoid any possible irritation of the scalp, you should ensure that Regaine has been removed by washing your hair and scalp before using these products.
You should inform your hair care professional that you are using Regaine.
Pregnancy, breastfeeding, and fertility
Regaine should not be used by women.
This medication is not recommended during pregnancy, breastfeeding, or when trying to become pregnant.
Driving and using machines
Regaine may cause dizziness or low blood pressure. If you experience these side effects, do not drive or operate machinery.
Regaine contains ethanol (alcohol)
This medication contains 564.6 mg of alcohol (ethanol) in each dose (1 gram) of cutaneous foam, which is equivalent to 564 mg/g (564.6% w/w). This may cause a burning sensation on damaged skin. If Regaine gets into your eyes, mouth, or a cut on your skin, rinse the area well with cold water.
Regaine contains butylhydroxytoluene
This medication may cause local skin reactions (e.g., contact dermatitis) or eye and mucous membrane irritation because it contains butylhydroxytoluene.
Regaine contains cetyl alcohol and stearyl alcohol
This medication may cause local skin reactions (contact dermatitis) because it contains cetyl alcohol and stearyl alcohol.
3. How to use Regaine
Follow the administration instructions for this medication as indicated in this package leaflet or as indicated by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
Use twice a day to achieve and maintain the desired effect of hair regrowth.
This foam is for use on the normal and healthy scalp only. DO NOT apply to other parts of the body.
Make sure to apply directly to the scalp.
Avoid contact with the eyes. If the foam gets into your eyes, rinse them with plenty of water.
Before using the foam:
Both the hair and scalp should be completely dry.
How to use the foam:
Apply a dose of 1 g (equivalent to half a capful) to the area of hair loss twice a day.
- To open the container, match the arrow on the cap ring with the arrow on the cap. Remove the cap (Figure 1).
- The foam may start to melt on contact with warm skin. If your fingers are warm, cool them first with water. Make sure to dry them before handling the foam.
- Hold the can upside down and press the nozzle to dispense the foam onto your fingertips (Figure 2). The total amount of foam applied should not exceed 1 g (equivalent to half a capful).
- Using your fingers, gently massage the foam into the affected areas of the scalp (Figure 3) and wash your hands immediately after applying the foam.
- After each use, close the container by putting the cap back on the can.
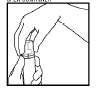
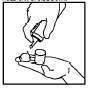
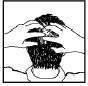
Figure 1 Figure 2 Figure 3
Duration of treatment with Regaine
Hair regrowth may take time to appear. Results may not appear until after two months of using the product twice a day. Some men may need to use the product for 4 months before observing results.
What to expect from using Regaine
In the first two months, some users have experienced an increase in hair shedding after using the foam for the first time. This may be a sign that Regaine is working. This temporary increase in hair shedding usually occurs between 2 and 6 weeks after starting treatment and then disappears.
If the increase in hair shedding continues after 6 weeks, you should consult your doctor.
Hair is normally soft and colorless. After subsequent applications, new hair should have the same color and thickness as the other hair on your head. Since hair grows slowly, it takes time to observe results.
Results may be seen in 2-4 months with two daily applications of the product.
If you do not observe hair regrowth after four months, you should stop using Regaine.
The amount of hair that regrows is different for each person. This product may not work for all men.
Continuous use is necessary to increase and maintain hair growth, or hair loss will begin again.
If you use more Regaine than you should
Follow the instructions for use. Do not use more than 2 g (equivalent to one capful) per day, and never more than twice a day. Hair will not grow faster, and you will not get better benefits if you use more or use it more frequently.
If a child swallows some Regaine, take them to the hospital immediately (with the Regaine can if possible, so the doctor can get an idea of how much the child has swallowed).
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20.
If you forget to use Regaine
Do not worry if you forget one or two applications, continue using the product as if you had not forgotten any application. Do not apply a double dose to make up for the forgotten dose, as this may cause low blood pressure, rapid heartbeat, and dizziness or fatigue.
If you stop treatment with Regaine
If you stop using the foam, hair regrowth may stop, and the scalp may return to its original state within 3-4 months.
If you have any doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Regaine can cause side effects, although not everyone will experience them.
STOP using the foam and contact your doctor immediately if you notice any of the following side effects.You may need urgent medical treatment.
- Swelling of the hands or feet (uncommon)
- Swelling of the face, lips, or throat, which can cause difficulty swallowing or breathing. This could be a sign of a severe allergic reaction (frequency not known, cannot be estimated from available data)
- Redness, itching, or persistent irritation of the scalp (not known)
- Weakness or dizziness (uncommon)
- Rapid heartbeat or irregular heartbeat (rare)
- Chest pain (rare)
- Low blood pressure (not known)
- Sudden and unexplained weight gain (common)
Other side effects that may occur:
- Common (may affect up to 1 in 10 people):
- Headache.
- Severe itching or skin rash.
- Shortness of breath or difficulty breathing
- Excessive hair growth
- Uncommon (may affect up to 1 in 100 people):
- Nausea.
Unknown side effects (whose frequency cannot be estimated from available data:
- Eye irritation
- Vomiting
- Temporary hair loss
- Changes in hair color and/or texture
- Administration site reactions, which can include itching, irritation, pain, rash, swelling, dry skin, and redness of the scalp, ears, or face, but can sometimes be more severe and include scaling, dermatitis, blistering, bleeding, and ulceration.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Agency's online platform: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Regaine
Keep this medication out of sight and reach of children.
Danger: extremely flammable aerosol. Pressurized container: May burst if heated. Keep away from heat sources, sparks, open flames, or hot surfaces, and other ignition sources. – Do not smoke. Do not spray near an open flame or other ignition source. Do not puncture or burn, even after use. Protect from sunlight, and store the container in its box. Do not expose to temperatures above 50°C.
Do not use this medication after the expiration date stated on the container after the letters "EXP". The expiration date is the last day of the month indicated.
Do not expose the container and its contents to flames during application. Medications should not be disposed of through wastewater or household waste. Deposit the containers and medications you no longer need at the pharmacy's SIGRE point. If you have any doubts, ask your pharmacist how to dispose of containers and medications you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Regaine
- The active ingredient is minoxidil. Each gram of cutaneous foam contains 50 mg of minoxidil.
- The other ingredients are anhydrous ethanol, purified water, butylhydroxytoluene (E321), lactic acid, anhydrous citric acid, glycerol, cetyl alcohol, stearyl alcohol, polysorbate 60, and propane/butane/isobutane (propellant)
Appearance and packaging of the product
This medication is presented as a cutaneous foam in a pressurized container with a child-resistant cap made of polypropylene. The foam is white to yellowish in color and odorless. It is contained in a 60 g (equivalent to 73 ml) container. The container contains enough for 1 month of treatment. The box may contain 1 or 3 containers.
Only certain pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
JNTL Consumer Health (Spain), S.L.
C/Vía de los Poblados 1, Edificio E, Planta 3
28033 Madrid - Spain
Manufacturer:JNTL CONSUMER HEALTH (FRANCE) S.A.S.
Domaine de Maigremont, 27100,
Val de Reuil, France
This medication has been authorized in the EEA Member States under the following names:
Finland, Norway, Sweden: Rogaine
Austria, Ireland, Romania, Slovenia, Spain: Regaine
France: Alostil
Date of the last revision of this package leaflet: March 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REGAINE 50 mg/g CUTANEOUS FOAMDosage form: TOPICAL SOLUTION, 50 mg/mlActive substance: minoxidilManufacturer: Mibe Pharma Espana S.L.Prescription not requiredDosage form: TOPICAL SOLUTION, 5%Active substance: minoxidilManufacturer: Laboratorios Vinas S.A.Prescription not requiredDosage form: TOPICAL SOLUTION, 50 mg/mlActive substance: minoxidilManufacturer: Laboratorios Serra Pamies S.A.Prescription not required
Online doctors for REGAINE 50 mg/g CUTANEOUS FOAM
Discuss questions about REGAINE 50 mg/g CUTANEOUS FOAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








