
ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА
Спросите врача о рецепте на ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА

Инструкция по применению ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА
Введение
Прошпект: информация для пациента
Протромплекс Тотал 600 UI / 20 мл
Порошок и растворитель для инъекционного раствора
комплекс протромбина человека
Прочитайте весь прошпект внимательно перед началомиспользованияэтого лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот прошпект, поскольку вам может потребоваться прочитать его снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этом прошпекте. См. раздел 4.
Содержание прошпекта
- Что такое Протромплекс Тотал и для чего он используется
- Что вам нужно знать перед началом использования Протромплекс Тотал
- Как использовать Протромплекс Тотал
- Возможные побочные эффекты
- Хранение Протромплекс Тотал
- Содержание упаковки и дополнительная информация
1. Что такое Протромплекс Тотал и для чего он используется
Протромплекс Тотал готовится из человеческой плазмы (жидкой части крови). Он содержит факторы свертывания крови II, VII, IX и X (факторы свертывания комплекса протромбина) и белок C.
Эти факторы свертывания зависят от витамина K и, как и витамин K, играют важную роль в свертывании крови. В случае дефицита одного из этих факторов кровь не свертывается так быстро, как обычно, что увеличивает склонность к кровотечению.
Протромплекс Тотал используется для:
- лечения кровотечений
- профилактики кровотечений непосредственно перед или после хирургической операции.
- приобретенный и врожденный дефицит факторов свертывания
Приобретенный дефицит:
Вы можете развить дефицит факторов свертывания, зависящих от витамина K (приобретенный дефицит), вызванный, например, лечением или передозировкой лекарств, которые снижают эффект витамина K (известных как антагонисты витамина K).
Врожденный дефицит:
Если вы родились с дефицитом (врожденный дефицит), это лекарство можно вводить непосредственно перед или после хирургической операции, когда не доступен концентрат соответствующего фактора.
2. Что вам нужно знать перед началом использования Протромплекс Тотал
Не используйте Протромплекс Тотал
- если вы аллергичны к факторам свертывания или к любому другому компоненту этого лекарства (указанному в разделе 6).
- если гепарин вызвал у вас или вы подозреваете, что он вызвал у вас снижение тромбоцитов, клеток, ответственных за свертывание крови (тромбоцитопения, индуцированная гепарином).
Предостережения и меры предосторожности
Трасабельность
Каждый раз, когда вам вводится доза Протромплекс Тотал, рекомендуется регистрировать название и номер партии продукта для поддержания контроля за использованными партиями.
Проконсультируйтесь с вашим врачом перед началом использования Протромплекс Тотал
- поскольку существует редкая возможность того, что вы можете развить тяжелую аллергическую реакцию (анафилактическую реакцию) на Протромплекс Тотал, поскольку были зарегистрированы аллергические реакции такого типа.
Вы можете найти подробную информацию о первых симптомах этого типа аллергической реакции в разделе 4 «Возможные побочные эффекты»
- если у вас есть приобретенный дефицит факторов свертывания, зависящих от витамина K.
Этот приобретенный дефицит может быть вызван лечением лекарствами, которые нейтрализуют свертывание крови путем ингибирования витамина K. В этом случае Протромплекс Тотал должен использоваться только тогда, когда необходима быстрая коррекция уровней комплекса протромбина, как в случаях тяжелых кровотечений или срочной хирургии. В других случаях достаточно снижения дозы антагониста витамина K или введения витамина K
- если вы принимаете лекарства для ингибирования свертывания крови (антагонисты витамина K). У вас может быть склонность к образованию тромбов, которая может увеличиться при введении концентрата комплекса протромбина человека
- если у вас есть врожденный дефицитнекоторых факторов свертывания, зависящих от витамина K, должен использоваться продукт с соответствующим фактором, если он доступен
- если вы проходите лечение концентратом комплекса протромбина человека, особенно если вы получали его повторно, поскольку могут образовываться тромбы (тромбоз) и попадать в кровоток (эмболия)
- если вы принадлежите к одной из следующих групп пациентов, из-за возможности образования тромбов:
- пациенты с коронарной болезнью сердца или перенесшие инфаркт миокарда
- пациенты с печеночной болезнью
- пациенты до или после операции
- новорожденные
- пациенты с риском тромбоэмболических осложнений или диссеминированной внутривенной коагуляции (ДВК)
Во всех этих ситуациях врач оценит тщательно пользу от лечения Протромплекс Тотал по сравнению с возможными рисками этих осложнений.
Безопасность вирусов
Когда вводятся лекарства, полученные из плазмы или человеческой крови, необходимо принимать определенные меры для предотвращения передачи инфекций пациентам.
Такие меры включают:
- тщательный отбор доноров для исключения тех, кто находится в группе риска быть носителями инфекционных заболеваний,
- анализ маркеров конкретных инфекций в индивидуальных донорских материалах и в плазменных смесях,
- включение стадий в процессе производства для удаления/инактивации вирусов.
Несмотря на это, когда вводятся лекарства, полученные из человеческой крови или плазмы, возможность передачи инфекционных агентов не может быть полностью исключена. Это также относится к возникающим вирусам или вирусам неизвестной природы или другим типам инфекций.
Эти меры считаются эффективными для вирусов с оболочкой, таких как вирус иммунодефицита человека (ВИЧ), вирус гепатита B и вирус гепатита C, и для необолоченного вируса гепатита A. Меры, принимаемые для других необолоченных вирусов, таких как парвовирус B19, могут иметь ограниченную ценность. Инфекция парвовирусом B19 может быть тяжелой для беременных женщин (инфекция плода) и для людей с ослабленной иммунной системой или пациентов с некоторыми видами анемии (например, серповидно-клеточной анемии или гемолитической анемии).
Возможно, ваш врач порекомендует вам вакцинацию против гепатита A и гепатита B, если вам вводится Протромплекс Тотал регулярно или повторно.
Рекомендуется вести учет введения каждой дозы Протромплекс Тотал, указывая название лекарства и номер партии, для поддержания регистра использованных партий.
Дети и подростки
Безопасность и эффективность использования Протромплекс Тотал у пациентов моложе 18 лет не были установлены в клинических испытаниях.
Другие лекарства и Протромплекс Тотал
Сообщите вашему врачу или фармацевту, если вы принимаете/используете, недавно принимали/использовали или можете принимать/использовать любое другое лекарство.
Сообщите вашему врачу, если вы принимаете лекарства для ингибирования свертывания крови (антагонисты витамина K). У вас может быть склонность к образованию тромбов, которая может увеличиться при введении концентрата комплекса протромбина человека.
Взаимодействие с биологическими тестами
Когда проводятся тесты на свертывание, чувствительные к гепарину, у пациентов, получающих высокие дозы комплекса протромбина человека, следует учитывать содержание гепарина в введенном продукте.
Беременность, лактация и фертильность
Если вы беременны или в период лактации, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства. Протромплекс Тотал можно использовать во время беременности и лактации только в тех случаях, когда это клинически указано.
Нет информации о влиянии Протромплекс Тотал на фертильность.
Вождение и использование машин
Не проводились исследования на способность вождения и использование машин.
Протромплекс Тотал содержит натрий и гепарин
Это лекарство содержит 81,7 мг натрия (основного компонента столовой соли/для приготовления пищи) в каждой ампуле. Это эквивалентно 4,1% от максимальной рекомендуемой суточной нормы потребления натрия для взрослого.
Гепарин может вызывать аллергические реакции и снижение количества кровяных клеток, которое может повлиять на систему свертывания крови. Пациенты с историей аллергических реакций, индуцированных гепарином, должны избегать использования лекарств, содержащих гепарин.
3. Как использовать Протромплекс Тотал
Начало, введение и наблюдение за лечением должны проводиться под строгим наблюдением врача с опытом лечения нарушений свертывания.
Доза и продолжительность лечения Протромплекс Тотал зависят от нескольких факторов, таких как вес тела, тяжесть заболевания, местоположение и интенсивность кровотечения или необходимости предотвращения кровотечения при хирургических процедурах.
Ваш врач рассчитает дозу в соответствии с вашими конкретными потребностями и будет регулярно контролировать свертывание и ваше клиническое состояние (см. раздел «Эта информация предназначена только для медицинских специалистов»).
Форма введения
Внутривенно.
Врач должен наблюдать за введением Протромплекс Тотал.
После восстановления с помощью стерильной воды для инъекций, поставляемой с Протромплекс Тотал, лекарство вводится медленно в вену (внутривенно). Скорость введения зависит от вашего самочувствия и не должна превышать 2 мл в минуту (60 ЕД/мин).
Использование у детей и подростков
Недостаточно данных для рекомендации введения Протромплекс Тотал пациентам моложе 18 лет.
Если вы использовали больше Протромплекс Тотал, чем необходимо
В случае передозировки увеличивается риск тромбоэмболических осложнений или коагулопатии потребления.
При введении высоких доз концентратов комплекса протромбина человека были зарегистрированы инфаркт миокарда, увеличение потребления тромбоцитов и факторов свертывания с повышенной формацией тромбов в кровеносных сосудах (коагулопатия потребления), тромбоз глубоких вен и эмболия легких.
Если у вас есть какие-либо другие вопросы об использовании этого лекарства, проконсультируйтесь с вашим врачом.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Как и при всех лечениях, полученных из плазмы, существует возможность развития аллергической реакции (анафилактической реакции). В отдельных случаях может развиться от тяжелой реакции гиперчувствительности до анафилактического шока.
Следовательно, вы должны обратить внимание на возможные ранние симптомы аллергической реакции, такие как:
- эритема (покраснение кожи)
- сыпь
- появление волдырей на коже (купероз/уртикария)
- зуд в любой части тела
- отек губ и языка
- затруднение дыхания/диспноэ
- сжатие в груди
- общее недомогание
- головокружение
- падение артериального давления
Если вы заметили один или несколько из этих симптомов, немедленно прекратите введение. Немедленно позвоните вашему врачу. Тяжелые симптомы требуют срочного лечения.
При использовании концентратов комплекса протромбина (включая Протромплекс Тотал) пациенты могут развить резистентность (ингибиторы) к одному или нескольким факторам свертывания, что приводит к инактивации факторов свертывания крови. Появление этих ингибиторов может проявляться как недостаточная реакция на лечение.
Во время лечения концентратами комплекса протромбина могут образовываться тромбы и попадать в кровоток, что может привести к осложнениям, таким как инфаркт миокарда, увеличение потребления тромбоцитов и факторов свертывания с повышенной формацией тромбов в кровеносных сосудах (коагулопатия потребления), тромбоз глубоких вен и эмболия легких.
Следующие побочные эффекты могут возникать у до 1 из 10 человек, использующих Протромплекс Тотал:
- образование тромбов в организме (диссеминированная внутривенная коагуляция), резистентность (ингибиторы) к одному или нескольким факторам комплекса протромбина (факторы II, VII, IX, X)
- тяжелая аллергическая реакция (анафилактический шок), анафилактическая реакция, гиперчувствительность, инсульт, головная боль
- инфаркт миокарда (острый инфаркт миокарда), тахикардия
- артериальный тромбоз, тромбоз глубоких вен, падение артериального давления (гипотония), покраснение кожи (рубор)
- эмболия легких, затруднение дыхания, одышка
- рвота, тошнота
- общая сыпь (уртикария), эритематозная сыпь, зуд (прурит)
- определенный почечный дисбаланс с симптомами, такими как отек век, лица и нижней части ног с увеличением веса, а также потеря белка с мочой (нефротический синдром)
- лихорадка (пирексия).
Следующие побочные эффекты были зарегистрированы при использовании других концентратов комплекса протромбина:
- отек лица, языка и губ (ангиоэдем), ощущение на коже, такое как жжение, покалывание, зуд или онемение (парестезия)
- реакция в месте инъекции
- летаргия
- беспокойство
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом, даже если это побочные эффекты, которые не указаны в этом прошпекте. Вы также можете сообщить об этом напрямую через Испанскую систему фармаковигиланса лекарств для человека: www.notificaRAM.es.
Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение Протромплекс Тотал
Хранить в холодильнике (при температуре от 2°C до 8°C). Не замораживать.
Хранить лекарство в оригинальной упаковке для защиты от света.
Держите это лекарство вне досягаемости детей.
Не используйте лекарство после даты истечения срока годности, указанной на упаковке после «CAD». Дата истечения срока годности — последний день месяца, указанного.
В течение срока годности продукт может храниться при комнатной температуре (макс. 25°C) в течение до 6 месяцев. Необходимо указать начало и конец хранения при комнатной температуре на упаковке лекарства.
После хранения при комнатной температуре Протромплекс Тотал не должен быть возвращен в холодильник (2°C — 8°C) и должен быть уничтожен, если не был использован в течение 6 месяцев.
Используйте раствор сразу после восстановления.
Лекарства не должны выбрасываться в канализацию или мусор. Спросите вашего фармацевта, как утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержимое упаковки и дополнительная информация
Состав Протромплекс Тотал
Порошок:
Активное вещество - комплекс протромбина человека, состоящий из человеческих факторов свертывания крови II, VII, IX и X и белка C.
Таблица:
На флакон МЕ | После восстановления с 20 мл стерильной воды для инъекций МЕ/мл | |
человеческий фактор II свертывания крови | –450-850 | –22,5-42,5 |
человеческий фактор VII свертывания крови | 500 | 25 |
человеческий фактор IX свертывания крови | 600 | 30 |
человеческий фактор X свертывания крови | 600 | 30 |
Один флакон содержит не менее 400 МЕ сопурифицированного белка C с факторами свертывания крови.
Другие компоненты: хлорид натрия, дигидрат цитрата натрия, натриевая гепарина (0,2-0,5 МЕ/МЕ фактора IX) и антитромбин III 15-30 МЕ на флакон (0,75-1,5 МЕ/мл).
Растворитель: Стерильная вода для инъекций.
Внешний видпродуктаи содержимое упаковки
Порошок и растворитель для инъекционной раствора.
Протромплекс Тотал - белый или слегка желтоватый порошок, лioфилизированный или в виде сухой компактной субстанции.
После восстановления раствор имеет pH между 6,5 и 7,5 и осмоляльность не ниже 240 мосм/кг. Раствор прозрачный или слегка опалесцирующий.
Порошок и растворитель находятся в флаконах из стекла для одноразового использования (гидролитическое стекло типа I и типа II соответственно) и запечатаны пробками из бутиловой резины.
Содержимое упаковки
1 флакон с Протромплекс Тотал 600 МЕ в порошке
1 флакон с 20 мл стерильной воды для инъекций
Согласно национальной маркировке, упаковки могут содержать следующие комбинации устройств:
-1 игла для переноса, 1 игла для аэрации, 1 игла для фильтра
-1 одноразовая шприц, 1 тройной набор (игла для аэрации, игла-бабочка и одноразовая игла), 1 игла для фильтра, 1 игла для переноса
-1 одноразовая шприц, 1 игла для аэрации, 1 игла-бабочка, 1 одноразовая игла, 1 игла для фильтра, 1 игла для переноса
-1 тройной набор (игла для аэрации, игла-бабочка и одноразовая игла), 1 игла для фильтра, 1 игла для переноса
-1 игла для переноса, 1 игла для фильтра, 1 игла для аэрации, 1 игла-бабочка, 1 одноразовая игла
-1 игла для переноса, 1 игла для фильтра, 1 одноразовая шприц, 1 игла для аэрации, 1 двойной набор (игла-бабочка, одноразовая игла)
Размер упаковки1 x 600 МЕ
Владелец разрешения на маркетинг и производитель
Владелец разрешения на маркетинг:
Baxalta Innovations GmbH
Industriestrasse 67
1221 Вена
Австрия
Производитель:
Takeda Manufacturing Austria AG
Industriestrasse, 67
A-1221 Вена, Австрия
Местный представитель:
Takeda Farmacéutica España S.A.
Calle Albacete, 5, planta 9ª
Edificio Los Cubos
28027 Мадрид
Испания
Тел: +34 91 790 42 22
Это лекарство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Австрия: | Протромплекс ТОТАЛ 600 ЕД. Порошок и растворитель для приготовления инъекционного раствора |
Бельгия, Люксембург: | Протромплекс 600 МЕ, порошок и растворитель для инъекционного раствора |
Болгария: | Протромплекс Тотал НФ 600 МЕ |
Чехия, Польша: | Протромплекс Тотал НФ |
Дания, Норвегия, Португалия: | Протромплекс |
Эстония, Греция: | Протромплекс ТОТАЛ |
Германия: | Протромплекс НФ 600 |
Венгрия: | Протромплекс ТОТАЛ 600 НЕ |
Ирландия, Мальта, Великобритания: | Протромплекс ТОТАЛ 600 МЕ |
Италия: | ПРОПЛЕКС |
Литва: | Протромплекс 600 ТВ порошок и растворитель для инъекционного раствора |
Латвия: | Протромплекс ТОТАЛ 600 СВ порошок и растворитель для приготовления инъекционного раствора |
Нидерланды: | Протромплекс 600 ИЕ порошок и растворитель для инъекционного раствора |
Румыния: | Протромплекс ТОТАЛ 600 МЕ порошок и растворитель для инъекционного раствора |
Словакия: | Протромплекс НФ 600 МЕ |
Словения: | ПРОПЛЕКС 600 ИЕ порошок и растворитель для инъекционного раствора |
Испания: | Протромплекс Тотал 600 МЕ |
Датапоследнего пересмотра этой инструкции:10/2024
Подробная и актуальная информация о этом лекарстве доступна на сайте Агентства по лекарственным средствам и медицинским изделиям Испании (AEMPS) http://www.aemps.gob.es/
-----------------------------------------------------------------------------------------------------------------------
Эта информация предназначена только для медицинских специалистов:
Дозировка
Ниже приведены общие рекомендации по дозировке, за исключением лечения кровотечений и профилактики кровотечений во время операций у пациентов, получающих антагонисты витамина К.
Лечение должно начинаться под наблюдением врача с опытом лечения нарушений свертывания крови. Доза и продолжительность лечения заместительной терапии зависят от тяжести нарушения, местоположения и интенсивности кровотечения, а также от клинического состояния пациента.
Количество и частота введения должны рассчитываться индивидуально для каждого пациента. Интервалы между дозами должны корректироваться в зависимости от различных периодов полувыведения различных факторов свертывания крови комплекса протромбина.
Индивидуальные требования к дозировке можно определить только на основе периодического определения уровня факторов свертывания крови в плазме или анализа общего уровня комплекса протромбина (например, теста Квика, МНО, времени протромбина) и постоянного наблюдения за клиническим состоянием пациента.
В случае проведения крупных хирургических операций необходимо проводить точный контроль заместительной терапии с помощью анализов свертывания крови (специфических тестов для факторов свертывания крови и/или общих анализов для определения уровня комплекса протромбина).
Кровотечение и профилактика кровотечений во время операций у пациентов, получающих антагонисты витамина К:
При тяжелых кровотечениях или перед операциями с высоким риском кровотечений необходимо достичь нормальных уровней (тест Квика 100%, МНО 1,0).
Применяется следующее правило: 1 МЕ фактора IX на килограмм веса тела увеличивает значение теста Квика примерно на 1%.
Если введение Протромплекс Тотал основано на значениях МНО, доза зависит от значения МНО до лечения и целевого значения МНО.
Необходимо следовать рекомендованным дозам, изложенным в следующей таблице, согласно публикациям Makris et al 20011.
Доза Протромплекс Тотал в зависимости от исходных значений МНО | |
МНО | Доза, МЕ/кг (МЕ относятся к фактору IX) |
2,0-3,9 | 25 |
4,0-6,0 | 35 |
>6,0 | 50 |
Коррекция антагониста витамина К, вызывающая нарушение гемостаза, сохраняется в течение примерно 6-8 часов. Однако эффекты витамина К, если он вводится одновременно, обычно достигаются в течение 4-6 часов. Следовательно, не необходимо повторять лечение комплексом протромбина человека, если введен витамин К.
Поскольку эти рекомендации являются эмпирическими, и восстановление и продолжительность действия могут варьироваться, обязательным является наблюдение за МНО во время лечения.
1Makris M, Watson HG: The Management of Coumarin-Induced Over-Anticoagulation Br. J. Haematol. 2011; 114:271-280
Лечение кровотечений и профилактика кровотечений во время операций при врожденной недостаточности одного из факторов свертывания крови, зависимых от витамина К, когда нет доступного очищенного продукта конкретного фактора свертывания:
Расчет необходимой дозы для лечения основан на эмпирических данных, что примерно 1 МЕ фактора VII или фактора IX на килограмм веса тела увеличивает активность плазменного фактора IX примерно на 0,015 МЕ/мл, и 1 МЕ фактора VII на килограмм веса тела увеличивает активность плазменного фактора VII примерно на 0,024 МЕ/мл. 1 МЕ фактора II или X на килограмм веса тела увеличивает активность плазменного фактора II или X примерно на 0,021 МЕ/мл2
2Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of Beriplex P/N prothrombin complex concentrate in healthy volunteers. Thromb Haemost. 2007;98(4):790-797
Доза конкретного фактора, введенного пациенту, выражается в Международных единицах (МЕ), которые связаны с текущим стандартом ВОЗ для каждого фактора. Активность плазменного фактора свертывания крови выражается либо в процентах (относительно нормальной плазмы), либо в Международных единицах (относительно международного стандарта для конкретного фактора свертывания).
Одна Международная единица (МЕ) активности фактора свертывания крови эквивалентна количеству, содержащемуся в 1 мл нормальной человеческой плазмы.
Необходимая доза определяется по следующей формуле:
Например, расчет необходимой дозы фактора X основан на эмпирических данных, что 1 МЕ фактора X на килограмм веса тела увеличивает активность плазменного фактора X примерно на 0,017 МЕ/мл. Необходимая доза определяется по следующей формуле:
Требуемые единицы = вес тела (кг) x желаемое увеличение фактора X (МЕ/мл) x 60
где 60 (мл/кг) является обратной величиной оценочного восстановления.
Если известно индивидуальное восстановление, это значение должно использоваться в расчете.
Педиатрическое население
Безопасность и эффективность применения Протромплекс Тотал у педиатрического населения не были установлены в клинических испытаниях Бакстер.
Взаимодействие с другими лекарственными средствами и другие формы взаимодействия
Если вводятся высокие дозы Протромплекс Тотал, необходимо учитывать содержание гепарина в продукте при проведении анализов свертывания крови, чувствительных к гепарину.
Несовместимости
Это лекарственное средство не должно смешиваться с другими лекарственными средствами, кроме растворителя, поставляемого с ним.
Как и все лекарственные средства факторов свертывания крови, эффективность и переносимость лекарства могут быть затронуты при смешивании с другими лекарственными средствами. Рекомендуется промыть общий венозный доступ физиологическим раствором до и после введения Протромплекс Тотал.
Особые меры предосторожности при утилизации и других манипуляциях
Для восстановления следует использовать только комплект для восстановления, поставляемый с препаратом.
Протромплекс Тотал должен быть восстановлен непосредственно перед введением. Раствор должен быть использован сразу после восстановления (раствор не содержит консервантов).
Раствор прозрачный или слегка опалесцирующий. Восстановленный продукт должен быть визуально осмотрен перед введением на отсутствие посторонних частиц или изменений цвета. Не использовать мутные растворы или содержащие осадки.
Восстановление порошка для инъекционного раствора:
Использовать асептическую технику!
- Нагреть флакон с растворителем (стерильную воду для инъекций) до комнатной температуры (макс. 37°C), не открывая.
- Снять защитные колпачки с флакона концентрата и флакона растворителя и продезинфицировать соответствующие резиновые пробки.
- Снять защитный колпачок с одного конца иглы для переноса, повернув и потянув, и вставить иглу в пробку флакона растворителя. (Рис. Б и В).
- Снять защитный колпачок с другого конца иглы для переноса, избегая прикосновения к открытому концу.
- Перевернуть флакон растворителя над флаконом порошка и вставить свободный конец иглы для переноса в пробку флакона порошка (Рис. Г). Растворитель будет засасываться в флакон порошка под действием вакуума.
- Отсоединить два флакона, удалив иглу для переноса вместе с флаконом растворителя из флакона порошка (Рис. Д). Аккуратно встряхнуть флакон концентрата, чтобы ускорить растворение.
- После полного растворения порошка вставить включенную иглу для аэрации (Рис. Е) и удалить возможную пену. Удалить иглу для аэрации.
Введение/инфузия:
Восстановленный продукт должен быть всегда визуально осмотрен перед введением на отсутствие посторонних частиц или изменений цвета.
Использовать асептическую технику!
- Снять защитный колпачок с включенной иглы для фильтра, повернув и потянув, и поместить иглу в стерильную одноразовую шприц. Ввести раствор в шприц (Рис. Ж).
- Отделить иглу для фильтра от шприца и медленно ввести раствор внутривенно (максимальная скорость введения/инфузии: 2 мл/мин).
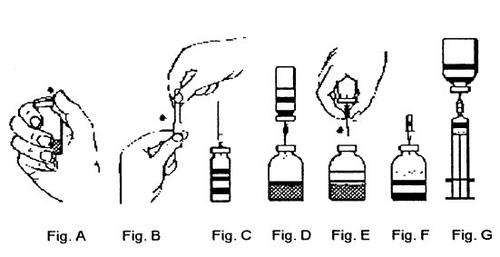
После введения утилизировать все открытые иглы вместе с шприцем и/или оборудованием для введения в упаковку продукта, чтобы избежать риска для других людей.
Утилизация неиспользованного лекарства и всех материалов, контактировавших с ним, должна осуществляться в соответствии с местными правилами.
Заведите запись о каждом введении Протромплекс Тотал в медицинскую карту пациента, используя прилагаемую самоклеящуюся этикетку.
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРАФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 1000 МЕ/флаконАктивное вещество: Coagulation factor IX, II, VII and X in combinationПроизводитель: Csl Behring GmbhТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 500 МЕАктивное вещество: Coagulation factor IX, II, VII and X in combinationПроизводитель: Csl Behring GmbhТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 250 МЕАктивное вещество: Coagulation factor IX, II, VII and X in combinationПроизводитель: Prothya Biosolutions Netherlands B.V.Требуется рецепт
Аналоги ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в Польша
Аналог ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в Украина
Врачи онлайн по ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ПРОТРОМПЛЕКС ТОТАЛ 600 МЕ / 20 мл ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА – по решению врача и с учетом местных правил.














