
БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА

Спросите врача о рецепте на БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА

Инструкция по применению БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА
Введение
ПРОСПЕКТ: ИНФОРМАЦИЯ ДЛЯ ПОЛЬЗОВАТЕЛЯ
Beriplex 1000 UI
Порошок и растворитель для инъекционной раствора
Человеческий протромбиновый комплекс
Прочитайте внимательно весь листок-вкладыш перед началом использования лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот листок-вкладыш, поскольку вам может потребоваться перечитать его.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено вам, и не следует давать его другим людям, даже если у них есть те же симптомы, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, сообщите об этом вашему врачу или фармацевту, даже если это побочные эффекты, которые не указаны в этом листке-вкладыше.
Содержание листка-вкладыша
- Что такое Beriplex и для чего он используется
- Что вам нужно знать перед началом использования Beriplex
- Как использовать Beriplex
- Возможные побочные эффекты
- Хранение Beriplex
- Содержание упаковки и дополнительная информация
1. Что такое Beriplex и для чего он используется
Что такое Beriplex
Beriplex выпускается в виде порошка и растворителя. Это белый или слегка окрашенный порошок или хрупкий твердый substance. Приготовленный раствор следует вводить путем инъекции в вену.
Beriplex приготовлен из человеческой плазмы (жидкой части крови) и содержит человеческие факторы свертывания II, VII, IX и X. Концентраты, содержащие эти факторы свертывания, называются продуктами протромбинового комплекса. Факторы свертывания II, VII, IX и X зависят от витамина K и важны для свертывания крови. Отсутствие одного из этих факторов означает, что кровь не свертывается с необходимой скоростью, и увеличивается склонность к кровотечению. Замена факторов II, VII, IX и X с помощью Beriplex восстанавливает механизмы свертывания.
Для чего используется Beriplex
Beriplex используется для профилактики (во время операции) и лечения кровотечений, вызванных приобретенной или врожденной недостаточностью факторов свертывания, зависящих от витамина K, т.е. факторов II, VII, IX и X в крови, когда нет доступа к очищенным продуктам конкретного фактора свертывания.
2. Что вам нужно знать перед началом использования Beriplex
Следующие разделы содержат информацию, которую ваш врач должен учитывать перед введением Beriplex.
Не используйте Beriplex
- Если вы аллергичны к активным веществам или любому другому компоненту этого лекарства (перечисленным в разделе 6).
Сообщите вашему врачу, если вы аллергичны к любому лекарству или продукту.
- Если вы более склонны к образованию кровяных сгустков, чем обычно (пациенты с риском диссеминированной внутривенной коагуляции).
- Если вы проявляете аллергическую реакцию на гепарин, вызывающую снижение количества тромбоцитов в крови (тромбоцитопения, индуцированная гепарином II типа, ТИГ II типа).
Сообщите вашему врачу или фармацевту, если вы страдаете одним из этих заболеваний.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед началом использования Beriplex в случае:
- Приобретенная недостаточность факторов свертывания, зависящих от витамина K: это может быть вызвано лекарствами, ингибирующими действие витамина K. Beriplex следует использовать только тогда, когда необходима быстрая коррекция уровня протромбинового комплекса, например, при тяжелых кровотечениях или срочной операции.
- Врожденная недостаточность любого из факторов, зависящих от витамина K: в этом случае следует использовать продукты конкретного фактора свертывания, если они доступны.
- Аллергические реакции или анафилактические реакции (тяжелая аллергическая реакция, вызывающая значительные трудности с дыханием или головокружение): следует немедленно прекратить введение Beriplex (например, прекратить инъекцию).
- Увеличенный риск образования кровяных сгустков в кровеносном сосуде (тромбоз), особенно:
- Если вы перенесли сердечный приступ (история коронарного сердечного заболевания или инфаркта миокарда).
- Если вы страдаете заболеванием печени.
- Если вы недавно были прооперированы (пациенты в пред- или послеоперационном периоде).
- У новорожденных (неонатов).
- Если вы более склонны к образованию кровяных сгустков, чем обычно (пациенты с риском тромбоэмболических эпизодов или диссеминированной внутривенной коагуляции или одновременной недостаточностью ингибитора коагуляции).
- Увеличенный риск коагуляции из-за повышенного потребления тромбоцитов или факторов свертывания крови. Лечение Beriplex можно начинать только после устранения основной причины.
- Снижение образования тромбоцитов из-за гепарина (тромбоцитопения, индуцированная гепарином, ТИГ II типа). Гепарин, белок, обладающий эффектом растворения кровяных сгустков, является компонентом Beriplex. Тяжелая форма снижения тромбоцитов может быть связана с
- образованием кровяных сгустков в вене или ноге,
- увеличением образования кровяных сгустков,
- в некоторых случаях с кожной сыпью в месте инъекции,
- петехиями (кровотечениями в виде точек) и
- черными каловыми массами (алвитранированными)
В этих случаях эффект гепарина может быть снижен (толерантность к гепарину). Если появляются такие симптомы, следует немедленно прекратить использование продукта и сообщить об этом вашему врачу. В будущем не следует использовать продукты, содержащие гепарин.
- Была зарегистрирована особая форма воспаления почек после лечения у пациентов с гемофилией B и ингибиторами фактора IX. Было известно, что эти пациенты имели историю аллергической реакции.
Ваш врач тщательно взвесит пользу лечения Beriplex и риск развития этих осложнений.
Вирусная безопасность
При приготовлении лекарств из человеческой крови или плазмы применяются определенные меры для предотвращения передачи инфекций пациентам. Эти меры включают:
- осторожный отбор доноров крови и плазмы для обеспечения исключения тех, кто представляет риск быть носителем инфекций,
- анализ каждой донорской крови и плазмы для обнаружения признаков вирусов или инфекций,
- включение этапов обработки крови или плазмы, которые могут инактивировать или удалить вирусы.
Несмотря на эти меры, при введении лекарств, приготовленных из человеческой крови или плазмы, нельзя полностью исключить возможность передачи инфекции. Это также верно для вирусов или других типов инфекций, неизвестных или возникающих.
Применяемые меры считаются эффективными для вирусов с оболочкой, таких как вирус иммунодефицита человека (ВИЧ), вирус гепатита B и вирус гепатита C (воспаление печени), и для необолоченного вируса гепатита A и парвовируса B19.
Возможно, ваш врач порекомендует вакцинацию против гепатита A и B, если вы регулярно или повторно лечитесь продуктами протромбинового комплекса, полученными из человеческой плазмы.
Рекомендуется вести точный учет каждого введения Beriplex, записывая название и номер партии лекарства, для ведения истории использованных партий.
Использование Beriplex с другими лекарствами
- Сообщите вашему врачу или фармацевту, если вы используете или最近 использовали или можете использовать любое другое лекарство.
- Beriplex может ингибировать действие антагониста витамина K. Не известны взаимодействия с другими лекарствами.
- Это лекарство не должно смешиваться с другими лекарствами, кроме тех, которые указаны в разделе 6.
Беременность, лактация и фертильность
- Если вы беременны или кормите грудью, или считаете, что могли стать беременной или планируете беременность, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства.
- Во время беременности и лактации Beriplex следует использовать только при явной необходимости.
- Нет данных о фертильности.
Вождение и использование машин
Не проводились исследования на способность вождения или использование машин.
Beriplex содержит натрий
Пациенты с диетами, бедными натрием, должны учитывать, что это лекарство содержит до 343 мг натрия (приблизительно 15 ммоль) на 100 мл. Это должно быть учтено пациентами, находящимися на контролируемой натриевой диете.
3. Как использовать Beriplex
Лечение должно быть начато и контролироваться врачом с опытом лечения этого типа расстройств.
Дозировка
Количество фактора II, VII, IX и X, которое вам необходимо, и продолжительность лечения будут зависеть от различных факторов, таких как ваш вес, тяжесть и характер вашего заболевания, местоположение и тяжесть кровотечения или необходимости предотвращения кровотечения во время операции или исследования (см. раздел “Эта информация предназначена только для медицинских специалистов”).
Если у вас есть какие-либо вопросы о использовании этого лекарства, проконсультируйтесь с вашим врачом или фармацевтом.
Передозировка
Ваш врач должен регулярно проверять состояние свертывания вашей крови во время лечения. Высокие дозы концентрата протромбинового комплекса были связаны с эпизодами сердечных приступов, диссеминированной внутривенной коагуляции и увеличением образования кровяных сгустков в кровеносных сосудах у пациентов с риском развития этих осложнений.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди их испытывают.
Следующие побочные эффекты были частонаблюдаемы (могут поражать до 1 из 10 пациентов):
- Существует риск образования кровяных сгустков (см. раздел 2).
- Головная боль.
- Повышение температуры тела.
Следующие побочные эффекты возникли с низкой частотой(могут поражать до 1 из 100 пациентов):
- Гиперчувствительность или аллергические реакции (см. раздел 2).
Частота следующих побочных эффектов является неизвестной(не может быть оценена с помощью доступных данных):
- Чрезмерная коагуляция, приводящая к тяжелому кровотечению.
- Анафилактические реакции, включая шок (см. раздел 2).
- Развитие циркулирующих антител, ингибирующих один или несколько факторов свертывания.
Педиатрическое население
Нет данных о использовании Beriplex у педиатрического населения.
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, сообщите об этом вашему врачу или фармацевту, даже если это побочные эффекты, которые не указаны в этом листке-вкладыше. Также можно сообщить об этом напрямую через Систему испанской фармаковигиланции для лекарств для человека: www.notificaRAM.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение BERIPLEX
- Храните это лекарство в недоступном для детей месте.
- Не используйте это лекарство после истечения срока годности, указанного на этикетке и упаковке после EXP.
- Не храните при температуре выше 25 °C.
- Не замораживайте.
- Храните упаковку в наружной упаковке для защиты от света.
- Beriplex не содержит консервантов, поэтому приготовленный раствор следует использовать немедленно.
Лекарства не следует выбрасывать в канализацию или мусор. В случае сомнений проконсультируйтесь с вашим фармацевтом о том, как утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Бериплекса
Бериплекс 1000 МЕ содержит 800 – 1240 МЕ человеческого коагуляционного фактора IX на флакон.
Активное вещество:
Концентрат человеческих коагуляционных факторов II, VII, IX и X, белков C и S.
Другие компоненты:
Человеческий антитромбин III, гепарин, человеческий альбумин, хлорид натрия, цитрат натрия, HCl или NaOH (в небольших количествах для регулирования pH).
Растворитель:Вода для инъекций.
Внешний вид продукта и содержание упаковки
Бериплекс представлен в виде белого или слегка окрашенного порошка и поставляется с водой для инъекций в качестве растворителя. Порошок должен быть растворен в 40 мл воды для инъекций.
Приготовленный раствор должен быть прозрачным или слегка опалесцирующим, т.е. может содержать пузырьки, когда его помещают перед светом, но не должен содержать обнаруживаемых частиц.
Презентация
Упаковка с 1000 МЕ, содержащая:
- 1 флакон с порошком
- 1 флакон с 40 мл воды для инъекций
- 1 трансферный набор с фильтром 20/20
Владелец разрешения на маркетинг и производитель
CSL Behring GmbH
Emil-von-Behring-Strasse 76
35041 Марбург
Германия
Для получения дополнительной информации об этом лекарственном средстве можно обратиться к местному представителю владельца разрешения на маркетинг:
CSL Behring, S.A.
c/ Tarragona 157, planta 18
08014 Барселона
Испания
Это лекарственное средство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Австрия | Бериплекс P/N 1000 ИЕ. Порошок и растворитель для приготовления инъекционного раствора |
Бельгия | Конфидекс 1000 ИЕ, порошок и растворитель для приготовления инъекционного раствора |
Болгария | Бериплекс P/N 1000, 1000 ИЕ, порошок и растворитель для приготовления инъекционного раствора |
Хорватия | Бериплекс P/N 1000 ИЕ порошок и растворитель для приготовления инъекционного раствора |
Чехия | Бериплекс 1000 ИЕ |
Дания | Конфидекс |
Финляндия | Конфидекс 1000 ИЕ инъекционный порошок и растворитель для приготовления раствора |
Франция | Конфидекс 1000 ИЕ, порошок и растворитель для приготовления инъекционного раствора |
Германия, Польша | Бериплекс P/N 1000 |
Греция | Бериплекс P/N Κ?νις и διαλ?της για εν?σιμο δι?λυμα 1000 ИЕ/флакон |
Венгрия | Бериплекс P/N 1000 порошок и растворитель для приготовления инъекционного раствора |
Италия | Конфидекс 1000 |
Люксембург | Конфидекс 1000 ИЕ порошок и растворитель для приготовления инъекционного раствора |
Мальта Нидерланды | Бериплекс P/N 1000, порошок и растворитель для приготовления инъекционного раствора Бериплекс P/N 1000 ИЕ, порошок и растворитель для приготовления инъекционного раствора |
Норвегия | Конфидекс 1000 ИЕ порошок и жидкость для приготовления инъекционного раствора |
Румыния Испания | Бериплекс P/N 1000 ИЕ порошок и растворитель для приготовления инъекционного раствора Бериплекс 1000 ИЕ порошок и растворитель для приготовления инъекционного раствора |
Швеция | Конфидекс 1000 ИЕ порошок и жидкость для приготовления инъекционного раствора |
Великобритания | Бериплекс P/N 1000 ИЕ, порошок и растворитель для приготовления инъекционного раствора |
Дата последнего пересмотра этого листка инструкции:Июль 2017
Подробная и актуальная информация о этом лекарственном средстве доступна на сайте Агентства по лекарственным средствам и медицинским изделиям Испании (AEMPS) http://www.aemps.gob.es
Эта информация предназначена исключительно для медицинских специалистов:
Качественный и количественный состав
Номинальное содержание человеческих коагуляционных факторов выражается в следующей таблице, выраженной в МЕ:
Название компонента | Содержание после реconstitution (МЕ/мл) | Бериплекс 1000 МЕсодержание на флакон (МЕ) |
Активные вещества | ||
Фактор II коагуляции, человеческий | 20 – 48 | 800 – 1920 |
Фактор VII коагуляции, человеческий | 10 – 25 | 400 – 1000 |
Фактор IX коагуляции, человеческий | 20 – 31 | 800 – 1240 |
Фактор X коагуляции, человеческий | 22 – 60 | 880 – 2400 |
Другие активные вещества | ||
Белок C | 15 – 45 | 600 – 1800 |
Белок S | 12 - 38 | 480 – 1520 |
Общее содержание белка составляет 6 – 14 мг/мл реconstituted раствора.
Специфическая активность, выраженная в факторе IX, составляет 2,5 МЕ/мг общего белка.
Активность всех коагуляционных факторов, а также белков C и S (антиген) была протестирована в соответствии с международными стандартами, установленными Всемирной организацией здравоохранения (ВОЗ).
Дозировка и метод введения
Дозировка
Следующие дозировочные рекомендации носят общий характер.
Дозировка и частота введения должны быть установлены индивидуально для каждого пациента. Интервалы дозирования должны быть адаптированы к периоду полувыведения соответствующих коагуляционных факторов комплекса протромбина. Индивидуальные дозировочные требования могут быть определены только на основе периодического определения плазменных уровней коагуляционных факторов или глобального анализа уровней комплекса протромбина (МНО, тест Квика) и постоянного мониторинга клинического состояния пациента.
В случае проведения крупных хирургических вмешательств необходим точный мониторинг заместительной терапии с помощью анализов коагуляции (специфических тестов коагуляционных факторов и/или глобальных тестов для измерения уровней комплекса протромбина).
- Кровотечение и профилактика перинатального кровотечения во время лечения антикоагулянтами витамина К.
Дозировка зависит от значения МНО до лечения и желаемого значения МНО. Значение МНО до лечения должно быть взято как можно ближе к моменту введения для расчета необходимой дозы Бериплекса. В следующей таблице приведены приблизительные дозы (мл реconstituted раствора/кг массы тела и МЕ фактора IX/кг массы тела), необходимые для нормализации МНО (например, <1,3) при различных исходных уровнях МНО.
МНО до лечения | 2,0 – 3,9 | 4,0 – 6,0 | > 6,0 |
Приблизительная доза (мл/кг массы тела) | 1 | 1,4 | 2 |
Приблизительная доза фактора IX (МЕ)/кг массы тела | 25 | 35 | 50 |
Доза основана на массе тела до, но не превышающей 100 кг. Для пациентов с массой тела более 100 кг максимальная единовая доза (МЕ фактора IX) не должна превышать 2500 МЕ для МНО 2,0 – 3,9, 3500 МЕ для МНО 4,0 – 6,0 и 5000 МЕ для МНО > 6,0.
Исправление нарушения гемостаза, индуцированного антикоагулянтами витамина К, обычно достигается примерно через 30 минут после инъекции. Одновременное введение витамина К должно быть рассмотрено у пациентов, леченных Бериплексом для срочной нейтрализации антикоагулянтов витамина К, поскольку эффект витамина К обычно достигается через 4-6 часов. Не рекомендуется повторное введение Бериплекса у пациентов, которым требуется срочное лечение нейтрализации антикоагулянтов витамина К, поскольку это не подтверждено клиническими данными.
Эти рекомендации основаны на данных клинических испытаний с ограниченным количеством лиц. Восстановление и продолжительность действия могут варьироваться, поэтому мониторинг МНО во время лечения является обязательным.
- Кровотечение и профилактика перинатального кровотечения при врожденной недостаточности некоторых коагуляционных факторов, зависимых от витамина К, когда нет доступа к специфическим препаратам коагуляционного фактора.
Расчет необходимой дозы концентрата комплекса протромбина основан на данных клинических испытаний:
?1 МЕ фактора IX на килограмм массы тела может увеличить плазменную активность фактора IX на 1,3% (0,013 МЕ/мл) от нормальной активности.
?1 МЕ фактора VII на килограмм массы тела увеличивает плазменную активность фактора VII на 1,7% (0,017 МЕ/мл) от нормальной активности.
?1 МЕ фактора II на килограмм массы тела увеличивает плазменную активность фактора II на 1,9% (0,019 МЕ/мл) от нормальной активности.
?1 МЕ фактора X на килограмм массы тела увеличивает плазменную активность фактора X на 1,9% (0,019 МЕ/мл) от нормальной активности.
Дозировка конкретного фактора, введенного, выражается в Международных единицах (МЕ), которые связаны со стандартом ВОЗ для каждого фактора. Плазменная активность конкретного коагуляционного фактора выражается либо в процентах (относительно нормальной плазмы), либо в Международных единицах (относительно международного стандарта для конкретного коагуляционного фактора).
Одна Международная единица (МЕ) активности коагуляционного фактора эквивалентна количеству, содержащемуся в 1 мл нормальной человеческой плазмы.
Например, расчет необходимой дозы фактора X основан на том, что 1 МЕ фактора X на килограмм массы тела увеличивает плазменную активность фактора X на 0,019 МЕ/мл.
Необходимая доза определяется по следующей формуле:
Требуемые единицы = масса тела [кг] x желаемое увеличение фактора X [МЕ/мл] x 53, где 53 (мл/кг) является обратным значением оцененного восстановления.
Следует учитывать, что расчет основан на данных от пациентов, получавших антикоагулянты витамина К. Расчет, основанный на данных от здоровых добровольцев, обеспечил бы меньшую оценку необходимой дозы.
Если известно индивидуальное восстановление, следует использовать это значение в расчете.
Доступна специфическая информация о продукте из клинических испытаний с здоровыми добровольцами (N = 15), нейтрализации антикоагулянтов витамина К в лечении тяжелых кровотечений или профилактике перинатального кровотечения (N = 98, N = 43).
Педиатрическое население
Еще не установлена безопасность и эффективность Бериплекса у детей и подростков с помощью контролируемых клинических испытаний.
Гериатрическое население
Дозировка и метод введения у пациентов старше 65 лет соответствуют общим рекомендациям.
Метод введения
Общие инструкции
- Раствор должен быть прозрачным или слегка опалесцирующим. После фильтрации/экстракции (см. ниже) и перед введением реconstituted продукт должен быть визуально осмотрен на наличие посторонних частиц и изменений цвета.
- Не использовать мутные растворы или содержащие остатки.
- Реconstitution и экстракция должны быть выполнены в асептических условиях.
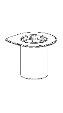
Реconstitution
Довести растворитель до комнатной температуры. Проверить, что были удалены уплотнения с флаконов продукта и растворителя, и что крышки были обработаны антисептическим раствором, дав им высохнуть перед открытием упаковки устройства Mix2Vial.
|
|
|
|
|
|
|
|
|
Утилизируйте флакон растворителя с прикрепленным синим адаптером Mix2Vial. |
|
|
|
|
Экстракция и введение
8 |
|
9 |
|
Следует быть осторожным, чтобы не допустить проникновения крови в шприц, загруженный продуктом, поскольку существует риск того, что кровь может свернуться в шприце и что с его помощью могут быть введены фибриновые сгустки пациенту.
Если необходимо более одного флакона Бериплекса, можно объединить несколько флаконов Бериплекса в одну инфузию с помощью коммерчески доступного инфузионного аксессуара.
Раствор Бериплекса не должен быть разбавлен.
Реconstituted раствор должен быть введен внутривенно (до 8 мл/минуту*).
Утилизация неиспользованного лекарственного средства и всех материалов, которые были в контакте с ним, должна быть выполнена в соответствии с местными правилами.
Особые предостережения и меры предосторожности
Нет данных о применении Бериплекса при перинатальном кровотечении, вызванном дефицитом витамина К у новорожденных.
Рекомендации для контроля количества тромбоцитов:
Тщательно контролируйте количество тромбоцитов.
Взаимодействие с другими лекарственными средствами и другие формы взаимодействия
При проведении тестов на коагуляцию, чувствительных к гепарину, у пациентов, леченных высокими дозами человеческого комплекса протромбина, следует учитывать содержание гепарина в введенном продукте.
* в клинических испытаниях с Бериплексом дозы вводились с максимальной скоростью инфузии 0,12 мл/кг/минуту у пациентов с весом <70 кг< p>
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРАФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 500 МЕАктивное вещество: Coagulation factor IX, II, VII and X in combinationПроизводитель: Csl Behring GmbhТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 250 МЕАктивное вещество: Coagulation factor IX, II, VII and X in combinationПроизводитель: Prothya Biosolutions Netherlands B.V.Требуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ, 500 МЕАктивное вещество: Coagulation factor IX, II, VII and X in combinationПроизводитель: Prothya Biosolutions Netherlands B.V.Требуется рецепт
Аналоги БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в Польша
Аналог БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА в Украина
Врачи онлайн по БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на БЕРИПЛЕКС 1000 МЕ ПОРОШОК И РАСТВОРИТЕЛЬ ДЛЯ ПРИГОТОВЛЕНИЯ ИНЪЕКЦИОННОГО РАСТВОРА – по решению врача и с учетом местных правил.




 1
1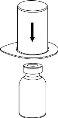 2
2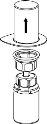 3
3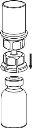 4
4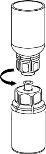 5
5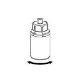 6
6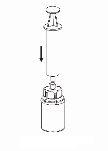 7
7









