
ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ

Спросите врача о рецепте на ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ

Инструкция по применению ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ
Введение
Прошпект: информация для пользователя
Леветирацетам Ауровитас 100 мг/мл раствор для приема внутрь ЕФГ
леветирацетам
Прочитайте внимательно весь прошпект перед тем, как вы или ваш ребенок начнете принимать это лекарство, поскольку в нем содержится важная информация для вас.
- Сохраните этот прошпект, поскольку вам может потребоваться прочитать его снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено только вам, и его не следует давать другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этом прошпекте. См. раздел 4.
Содержание прошпекта
- Что такое Леветирацетам Ауровитас и для чего он используется
- Что нужно знать перед началом приема Леветирацетама Ауровитас
- Как принимать Леветирацетам Ауровитас
- Возможные побочные эффекты
- Хранение Леветирацетама Ауровитас
- Содержание упаковки и дополнительная информация
1. Что такое Леветирацетам Ауровитас и для чего он используется
Леветирацетам является противоэпилептическим препаратом (лекарством для лечения приступов при эпилепсии).
Леветирацетам Ауровитас используется:
- в качестве монотерапии у взрослых и подростков в возрасте 16 лет и старше с недавно диагностированной эпилепсией для лечения одной формы эпилепсии. Эпилепсия - это заболевание, при котором пациенты испытывают приступы (приступы). Леветирацетам используется для лечения формы эпилепсии, при которой приступы изначально поражают только одну сторону мозга, но могут затем распространиться на более обширные области в обоих полушариях мозга (приступы с парциальным началом с или без вторичной генерализации). Ваш врач назначил леветирацетам, чтобы уменьшить количество приступов.
- в комбинации с другими противоэпилептическими препаратами для лечения:
- приступов с парциальным началом с или без генерализации у взрослых, подростков, детей и младенцев в возрасте от 1 месяца.
- миоклонических приступов (коротких, похожих на удар, сокращений мышц или группы мышц) у взрослых и подростков в возрасте от 12 лет с ювенильной миоклонической эпилепсией.
- примарных генерализованных тónico-клонических приступов (больших приступов, включая потерю сознания) у взрослых и подростков в возрасте от 12 лет с идиопатической генерализованной эпилепсией (типом эпилепсии, который, как считается, имеет генетическую причину).
2. Что нужно знать перед началом приема Леветирацетама Ауровитас
Не принимайтеЛеветирацетам Ауровитас
- Если вы аллергичны к леветирацетаму, производным пириролидона или любому другому компоненту этого препарата (перечисленному в разделе 6).
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом перед началом приема леветирацетама:
- Если у вас есть проблемы с почками, следуйте инструкциям вашего врача, который решит, нужно ли корректировать дозу.
- Если вы заметите любое снижение роста вашего ребенка или неожиданное развитие полового созревания, свяжитесь с вашим врачом.
- Небольшое количество людей, принимающих противоэпилептические препараты, такие как леветирацетам, испытывали мысли о причинении вреда себе или суицидальные мысли. Если у вас есть какие-либо симптомы депрессии и/или суицидальные мысли, свяжитесь с вашим врачом.
- Если у вас есть семейная или медицинская история аритмии (видимой на электрокардиограмме) или если у вас есть заболевание и/или вы принимаете лечение, которое делает вас склонным к аритмии или нарушениям баланса электролитов.
Сообщите вашему врачу или фармацевту, если любой из следующих побочных эффектов ухудшается или длится более нескольких дней:
- Аномальные мысли, чувство раздражительности или агрессивности или если вы или ваша семья и друзья заметили значительные изменения в вашем настроении или поведении.
- Ухудшение эпилепсии
В редких случаях приступы могут ухудшаться или возникать чаще, в основном в течение первого месяца после начала лечения или увеличения дозы.
В очень редкой форме ранней эпилепсии (эпилепсии, связанной с мутациями SCN8A), которая вызывает множественные типы приступов и потерю навыков, вы можете заметить, что приступы продолжаются или ухудшаются во время лечения.
Если вы испытываете любой из этих новых симптомов во время приема леветирацетама, обратитесь к врачу как можно скорее.
Дети и подростки
- Монотерапия леветирацетамом не показана у детей и подростков в возрасте до 16 лет.
Применение Леветирацетама Ауровитас с другими препаратами
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принять любой другой препарат.
Не принимайте макрогол (лекарство, используемое как слабительное) в течение часа до и после приема леветирацетама, поскольку это может уменьшить его эффект.
Беременность, лактация и фертильность
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом перед использованием этого препарата.
Леветирацетам можно использовать во время беременности только после тщательной оценки, если ваш врач считает это необходимым. Не прекращайте лечение без предварительной консультации с вашим врачом. Не можно полностью исключить риск врожденных дефектов у ребенка.
Грудное вскармливание не рекомендуется во время лечения.
Вождение и использование машин
Леветирацетам может изменить вашу способность управлять транспортными средствами или работать с инструментами или машинами, поскольку он может вызывать сонливость. Это более вероятно в начале лечения или при увеличении дозы. Не следует управлять транспортными средствами или работать с машинами до тех пор, пока не будет проверено, что ваша способность выполнять эти действия не нарушена.
Леветирацетам Ауровитассодержит метилпараоксибензоат (Е 218) и пропилпараоксибензоат (Е 216)
Леветирацетам раствор для приема внутрь, содержащий метилпараоксибензоат (Е218) и пропилпараоксибензоат (Е216), может вызывать аллергические реакции (возможно, задержанные).
Леветирацетам раствор для приема внутрь также содержит мальтитол. Если ваш врач сообщит вам, что у вас есть непереносимость некоторых сахаров, проконсультируйтесь с вашим врачом перед приемом этого препарата.
Пропиленгликоль
Этот препарат содержит 20,27 мг пропиленгликоля в каждом мл раствора для приема внутрь. Если ребенок имеет менее 4 недель, проконсультируйтесь с вашим врачом или фармацевтом, особенно если ребенку были назначены другие препараты, содержащие пропиленгликоль или алкоголь.
Леветирацетам Ауровитас 100 мг/мл раствор для приема внутрь ЕФГ содержит натрий
Этот препарат содержит менее 1 ммоль натрия (23 мг) на мл; это означает, что он практически не содержит натрия.
3. Как принимать Леветирацетам Ауровитас
Следуйте точно инструкциям по применению этого препарата, указанным вашим врачом или фармацевтом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом.
Леветирацетам следует принимать дважды в день, один раз утром и один раз вечером, примерно в одно и то же время каждый день.
Принимайте раствор для приема внутрь согласно инструкциям вашего врача.
Монотерапия(с 16 лет)
Взрослые (≥ 18 лет) и подростки (с 16 лет):
Измерьте подходящую дозу с помощью шприца на 10 мл, включенного в упаковку для пациентов в возрасте от 4 лет.
Рекомендуемая доза: Леветирацетам принимается дважды в день, разделенный на две равные дозы, каждая из которых составляет от 5 мл (500 мг) до 15 мл (1500 мг).
Когда вы начинаете принимать леветирацетам, ваш врач назначит нижнюю дозув течение двух недель перед назначением минимальной суточной дозы.
Сочетанная терапия
Доза для взрослых и подростков (от 12 до 17 лет):
Измерьте подходящую дозу с помощью шприца на 10 мл, включенного в упаковку для пациентов в возрасте от 4 лет.
Рекомендуемая доза:
Леветирацетам принимается дважды в день, разделенный на две равные дозы, каждая из которых составляет от 5 мл (500 мг) до 15 мл (1500 мг).
Доза длядетей в возрасте 6 месяцев и старше:
Ваш врач назначит наиболее подходящую лекарственную форму леветирацетама в зависимости от возраста, веса и дозы.
Для детей от 6 месяцев до 4 лет, измерьте подходящую дозу с помощью шприца на 3 мл, включенного в упаковку.
Для детей старше 4 лет, измерьте подходящую дозу с помощью шприца на 10 мл, включенного в упаковку.
Рекомендуемая доза: Леветирацетам принимается дважды в день, разделенный на две равные дозы, каждая из которых составляет от 0,1 мл (10 мг) до 0,3 мл (30 мг) на килограмм веса ребенка (см. примеры доз в таблице ниже).
Доза для детей в возрасте 6 месяцев и старше:
Вес | Начальная доза: 0,1 мл/кг дважды в день | Максимальная доза: 0,3 мл/кг дважды в день |
6 кг | 0,6 мл дважды в день | 1,8 мл дважды в день |
8 кг | 0,8 мл дважды в день | 2,4 мл дважды в день |
10 кг | 1 мл дважды в день | 3 мл дважды в день |
15 кг | 1,5 мл дважды в день | 4,5 мл дважды в день |
20 кг | 2 мл дважды в день | 6 мл дважды в день |
25 кг | 2,5 мл дважды в день | 7,5 мл дважды в день |
50 кг и более | 5 мл дважды в день | 15 мл дважды в день |
Дозирование у младенцев (от 1 месяца до менее 6 месяцев):
Для детей от 1 месяца до менее 6 месяцев, измерьте подходящую дозу с помощью шприца на 1 мл, включенного в упаковку.
Рекомендуемая доза: Леветирацетам принимается дважды в день, разделенный на две равные дозы, каждая из которых составляет от 0,07 мл (7 мг) до 0,21 мл (21 мг) на килограмм веса младенца (см. примеры доз в таблице ниже).
Дозирование у младенцев (от 1 месяца до менее 6 месяцев):
Вес | Начальная доза: 0,07 мл/кг дважды в день | Максимальная доза: 0,21 мл/кг дважды в день |
4 кг | 0,3 мл дважды в день | 0,85 мл дважды в день |
5 кг | 0,35 мл дважды в день | 1,05 мл дважды в день |
6 кг | 0,45 мл дважды в день | 1,25 мл дважды в день |
7 кг | 0,5 мл дважды в день | 1,5 мл дважды в день |
Способ применения:
После измерения правильной дозы с помощью подходящего шприца леветирацетам можно принимать, разбавляя раствор для приема внутрь в стакане воды или в бутылке. Леветирацетам можно принимать с пищей или без нее. После перорального приема леветирацетама может ощущаться горький вкус.
Инструкции для правильного применения:
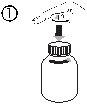 Открыть флакон: сжать пробку и повернуть в противоположном направлении часовой стрелки (рисунок 1)
Открыть флакон: сжать пробку и повернуть в противоположном направлении часовой стрелки (рисунок 1)
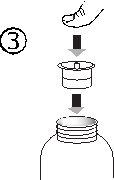
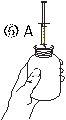
- Отделить адаптер шприца (рисунок 2). Вставить адаптер в горлышко флакона (рисунок 3). Убедиться, что он хорошо закреплен.
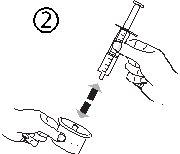
- Взять шприц и вставить его в отверстие адаптера (рисунок 4). Положить флакон вниз (рисунок 5).
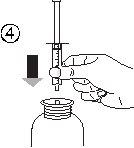
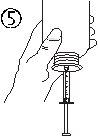
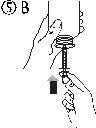
- Наполнить шприц небольшим количеством раствора, опустив поршень (рисунок 5А), а затем подняв его, чтобы удалить любые возможные пузырьки (рисунок 5Б). Опустить поршень до метки градуировки, соответствующей назначенной вашему врачу дозе в мл (рисунок 5В).
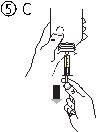
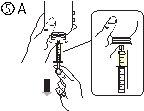
- Положить флакон вверх (рисунок 6А). Удалить шприц из адаптера (рисунок 6Б).
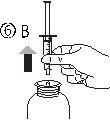
- Выпустить содержимое шприца в стакан воды или в бутылку, опустив поршень до конца шприца (рисунок 7).
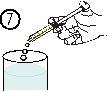
- Выпить содержимое стакана или бутылки полностью.
- Закрыть флакон с помощью пластиковой резиновой пробки.
- Вымыть шприц только водой (рисунок 8).
Продолжительность лечения:
- Леветирацетам используется как постоянное лечение. Вы должны продолжать лечение леветирацетамом в течение времени, указанного вашим врачом.
- Не прекращайте лечение без рекомендации вашего врача, поскольку это может увеличить количество приступов.
Если вы приняли больше Леветирацетама Ауровитас, чем следует
Возможные побочные эффекты передозировки леветирацетама - сонливость, агитация, агрессивность, снижение бдительности, угнетение дыхания и кома.
Свяжитесь с вашим врачом, если вы приняли больше раствора для приема внутрь, чем следует. Ваш врач определит лучшее возможное лечение передозировки.
Если вы пропустили прием Леветирацетама Ауровитас:
Свяжитесь с вашим врачом, если вы пропустили одну или несколько доз. Не принимайте двойную дозу, чтобы компенсировать пропущенные дозы.
Если вы прекратили лечение Леветирацетамом Ауровитас:
Прекращение лечения леветирацетамом должно осуществляться постепенно, чтобы избежать увеличения количества приступов. Если ваш врач решит прекратить ваше лечение леветирацетамом, он/она даст вам инструкции для постепенного отмены леветирацетама.
Если у вас есть какие-либо другие вопросы о применении этого препарата, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все лекарства, леветирацетам может вызывать побочные эффекты, хотя не все люди испытывают их.
Сообщите своему врачу немедленно или обратитесь в отделение неотложной помощи ближайшей больницы, если вы испытываете:
- слабость, головокружение или затруднение дыхания, поскольку это могут быть признаки тяжелой аллергической реакции (анафилактической);
- отек лица, губ, языка или горла (отек Квинке);
- симптомы гриппа и высыпания на лице, за которыми следует длительная высыпание с высокой температурой, повышенными уровнями печеночных ферментов в крови и увеличением количества определенных типов белых кровяных клеток (эозинофилия), увеличением лимфатических узлов и поражением других органов тела (реакция гиперчувствительности к лекарству с эозинофилией и системными симптомами (DRESS));
- симптомы, такие как низкий объем мочи, усталость, тошнота, рвота, спутанность сознания и отек ног, рук или ног, поскольку это может быть признаком внезапного снижения функции почек;
- кожная сыпь, которая может образовывать пузыри и выглядеть как небольшие мишени (центральные темные точки, окруженные более светлой областью, с темным кольцом вокруг края) (эритема многоформная);
- общая сыпь с пузырями и шелушением кожи, особенно вокруг рта, носа, глаз и гениталий (синдром Стивенса-Джонсона);
- более тяжелая форма, вызывающая шелушение кожи на более чем 30% поверхности тела (токсический эпидермальный некролиз);
- признаки тяжелых изменений психики или если кто-то вокруг вас заметил признаки спутанности сознания, сонливости (заторможенности), амнезии (потери памяти), ухудшения памяти (забывчивости), аномального поведения или других неврологических симптомов, включая непроизвольные или неконтролируемые движения. Это могут быть симптомы энцефалопатии.
Наиболее часто сообщаемые побочные эффекты - ринофарингит, сонливость (чувство сна), головная боль, усталость и головокружение. Побочные эффекты, такие как чувство сна, чувство слабости и головокружение, могут быть более частыми при начале лечения или увеличении дозы. Однако эти побочные эффекты должны уменьшаться со временем.
Очень часто: могут поражать более 1 из 10 человек
- ринофарингит;
- сонливость (чувство сна), головная боль.
Часто: могут поражать до 1 из 10 человек
- анорексия (потеря аппетита);
- депрессия, враждебность или агрессивность, тревога, бессонница, нервозность или раздражительность;
- припадки, нарушение равновесия, головокружение (чувство неустойчивости), летаргия (отсутствие энергии и энтузиазма), тремор (непроизвольный тремор);
- вертиго (чувство вращения);
- кашель;
- боль в животе, диарея, диспепсия (изжога), рвота, тошнота;
- кожная сыпь;
- астения/усталость (чувство слабости).
Не часто: могут поражать до 1 из 100 человек
- уменьшение количества тромбоцитов, уменьшение количества белых кровяных клеток;
- потеря веса, набор веса;
- попытка самоубийства и мысли о самоубийстве, изменения психики, аномальное поведение, галлюцинации, гнев, спутанность сознания, паническая атака, эмоциональная нестабильность/изменения настроения, агитация;
- амнезия (потеря памяти), ухудшение памяти (потеря памяти), аномальная координация/атаксия (нарушение координации движений), парестезия (ползание), нарушения внимания (потеря концентрации);
- диплопия (двойное зрение), размытое зрение;
- повышенные/анормальные значения в тестах на функцию печени;
- потеря волос, экзема, зуд;
- слабость мышц, миалгия (боль в мышцах);
- травма.
Редко: могут поражать до 1 из 1 000 человек
- инфекция;
- уменьшение всех типов кровяных клеток;
- тяжелые аллергические реакции (DRESS, анафилактическая реакция (тяжелая и серьезная аллергическая реакция), отек Квинке (отек лица, губ, языка и горла));
- уменьшение концентрации натрия в крови;
- самоубийство, расстройства личности (проблемы поведения), аномальное мышление (медленное мышление, трудности с концентрацией);
- делирий;
- энцефалопатия (см. подраздел "Сообщите своему врачу немедленно" для подробного описания симптомов);
- припадки могут ухудшаться или возникать чаще;
- неконтролируемые мышечные спазмы, поражающие голову, туловище и конечности, трудности с контролем движений, гиперкинезия (гиперактивность);
- изменение сердечного ритма (электрокардиограмма);
- панкреатит (воспаление поджелудочной железы);
- печеночная недостаточность, гепатит (воспаление печени);
- внезапное снижение функции почек;
- кожная сыпь, которая может привести к образованию пузырей и выглядеть как небольшие мишени (центральные темные точки, окруженные более светлой областью, с темным кольцом вокруг края) (эритема многоформная), общая сыпь с пузырями и шелушением кожи, особенно вокруг рта, носа, глаз и гениталий (синдром Стивенса-Джонсона) и более тяжелая форма, вызывающая шелушение кожи на более чем 30% поверхности тела (токсический эпидермальный некролиз);
- рабдомиолиз (разрушение мышечной ткани) и увеличение креатинфосфокиназы в крови. Распространенность значительно выше у японских пациентов по сравнению с неяпонскими пациентами;
- хромота или трудности с ходьбой;
- комбинация лихорадки, мышечной жесткости, нестабильного артериального давления и частоты сердечных сокращений, спутанности сознания, снижения уровня сознания (могут быть признаками расстройства, называемого синдромом нейролептической мальигнации). Распространенность значительно выше у японских пациентов по сравнению с неяпонскими пациентами.
Очень редко: могут поражать до 1 из 10 000 человек
- мысли или чувства, которые не желательны и повторяются, или импульс делать что-то снова и снова (обсессивно-компульсивное расстройство).
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не указаны в этом листке. Вы также можете сообщить о них напрямую через систему фармаковигиланса Испании: https://www.notificaram.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более подробной информации о безопасности этого лекарства.
5. Хранение Леветирацетама Ауровитас
Храните это лекарство в недоступном для детей месте.
Не используйте это лекарство после даты истечения срока годности, указанной на упаковке после CAD. Дата истечения срока годности - последний день месяца, указанного.
Это лекарство не требует специальных условий хранения.
Не используйте после 7 месяцев после открытия упаковки.
Лекарства не должны выбрасываться в канализацию или мусор. Положите упаковку и лекарства, которые вам больше не нужны, в специальный пункт сбора в аптеке. Если у вас есть сомнения, спросите у вашего фармацевта, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Леветирацетама Ауровитас
- Активное вещество - леветирацетам. Каждый мл содержит 100 мг леветирацетама.
- Другие компоненты - жидкий мальтитол (Е-965), глицерин (Е-422), пропиленгликоль, метилпараоксибензоат (Е-218), пропилпараоксибензоат (Е-216), цитриновая кислота моногидрат, цитрат натрия, ацесульфам калия (Е-950), Мафко Магнасвит (глицерин, глицирризинат аммония), вкус винограда (ароматизаторы, пропиленгликоль, аскорбиновая кислота), очищенная вода.
Внешний вид продукта и содержание упаковки
Леветирацетам Ауровитас 100 мг/мл пероральная растворенная форма является прозрачной, бесцветной жидкостью с вкусом винограда.
Стеклянная бутылка объемом 300 мл (тип III) Леветирацетама Ауровитас (для детей от 4 лет, подростков и взрослых) с белой крышкой, защищенной от детей, поставляется в картонной коробке с дозировочной ложкой объемом 10 мл (градуированной каждые 0,25 мл) и адаптером для ложки.
Стеклянная бутылка объемом 150 мл (тип III) Леветирацетама Ауровитас (для младенцев и маленьких детей от 6 месяцев до 4 лет) с белой крышкой, защищенной от детей, поставляется в картонной коробке с дозировочной ложкой объемом 3 мл (градуированной каждые 0,1 мл) и адаптером для ложки.
Стеклянная бутылка объемом 150 мл (тип III) Леветирацетама Ауровитас (для младенцев от 1 месяца до 6 месяцев) с белой крышкой, защищенной от детей, поставляется в картонной коробке с дозировочной ложкой объемом 1 мл (градуированной каждые 0,05 мл) и адаптером для ложки.
Возможно, не все размеры упаковки будут продаваться.
Владелец разрешения на маркетинг и производитель
Владелец разрешения на маркетинг:
Ауровитас Испания, С.А.У.
Авенида де Бургос, 16-Д
28036 Мадрид
Испания
Производитель:
АПЛ Свит Сервисес (Мальта) Лимитед
ХФ26, Хал Фар Индастриал Эстейт, Хал Фар
Бирзеббуджа, ББГ 3000
Мальта
Это лекарство разрешено к маркетингу в государствах-членах Европейского экономического пространства под следующими названиями:
Испания: Леветирацетам Ауровитас 100 мг/мл пероральная растворенная форма
Италия: Леветирацетам Ауробиндо Фарма Италия
Мальта: Леветирацетам Ауробиндо 100 мг/мл пероральный раствор
Португалия: Леветирацетам Ауровитас
Дата последнего пересмотра этого листка:апрель 2025
Подробная информация о этом лекарстве доступна на сайте Агентства по лекарствам и медицинским продуктам Испании (АЕМПС) (http://www.aemps.gob.es)

Сколько стоит ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ в Испании в 2025 году?
Средняя цена на ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ в декабрь, 2025 года составляет около 56.64 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках56.64 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР ДЛЯ ИНФУЗИЙ, 100 мгАктивное вещество: ЛеветирацетамПроизводитель: Ucb PharmaТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР ДЛЯ ИНФУЗИЙ, 100 мг/млАктивное вещество: ЛеветирацетамПроизводитель: Ucb PharmaТребуется рецептФорма выпуска: ОРАЛЬНЫЙ РАСТВОР/СУСПЕНЗИЯ, 100 мгАктивное вещество: ЛеветирацетамПроизводитель: Ucb PharmaТребуется рецепт
Аналоги ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ в Polonia
Аналог ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ в Ucrania
Врачи онлайн по ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ЛЕВЕТИРАЦЕТАМ АУРОВИТАС 100 мг/мл РАСТВОР ДЛЯ ПРИЕМА ВНУТРИ – по решению врача и с учетом местных правил.







