
Инструкция по применению ЛЕНАЛИДОМИД Ауровитас 15 мг ТВЕРДЫЕ КАПСУЛЫ
Введение
Прошпект: Информация для пользователя
Леналидомида Ауровитас 5 мг твердые капсулы ЕФГ
Леналидомида Ауровитас 10 мг твердые капсулы ЕФГ
Леналидомида Ауровитас 15 мг твердые капсулы ЕФГ
Леналидомида Ауровитас 20 мг твердые капсулы ЕФГ
Леналидомида Ауровитас 25 мг твердые капсулы ЕФГ
Прочитайте весь прошпект внимательно перед началом приема этого лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот прошпект, поскольку вам может потребоваться перечитать его.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено только вам, и его не следует давать другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этом прошпекте. См. раздел 4.
Содержание прошпекта
- Что такое Леналидомида Ауровитас и для чего он используется
- Что нужно знать перед началом приема Леналидомида Ауровитас
- Как принимать Леналидомида Ауровитас
- Возможные побочные эффекты
- Хранение Леналидомида Ауровитас
- Содержание упаковки и дополнительная информация
1. Что такое Леналидомида Ауровитас и для чего он используется
Что такое Леналидомида Ауровитас
Леналидомида Ауровитас содержит активное вещество "леналидомида". Это лекарство относится к группе лекарств, которые влияют на то, как работает иммунная система.
Для чего используется Леналидомида Ауровитас
Леналидомида используется у взрослых для:
- Многочисленной миеломы
- Миелодиспластических синдромов (МДС)
- Лимфомы мантийных клеток (ЛМК)
- Фолликулярного лимфомы
Многочисленная миелома
Многочисленная миелома - это тип рака, который поражает определенный тип белых кровяных клеток, называемых плазматическими клетками. Эти клетки накапливаются в костном мозге и размножаются, выходя из-под контроля. Это может повредить кости и почки.
Многочисленная миелома, как правило, не имеет лечения. Однако можно значительно уменьшить симптомы или сделать их исчезнуть на некоторое время. Это называется "ремиссией".
Миелома нового диагноза: у пациентов, которые прошли трансплантацию костного мозга
Леналидомида используется как поддерживающее лечение после адекватного восстановления после трансплантации костного мозга.
Многочисленная миелома нового диагноза: у пациентов, которые не могут быть behandены трансплантацией костного мозга
Леналидомида принимается с другими лекарствами, включая:
- химиотерапевтическое лекарство под названием "бортезомиб"
- противовоспалительное лекарство под названием "дексаметазон"
- химиотерапевтическое лекарство под названием "мелфалан" и
- иммунодепрессивное лекарство под названием "преднизон"
Вы будете принимать эти лекарства в начале лечения, а затем продолжите принимать леналидомида в одиночку.
Если вам 75 лет или более, или у вас есть проблемы с почками средней или тяжелой степени, ваш врач будет внимательно следить за вами перед началом лечения.
Многочисленная миелома: у пациентов, которые ранее получали лечение
Леналидомида принимается вместе с противовоспалительным лекарством под названием "дексаметазон".
Леналидомида может замедлить ухудшение симптомов многочисленной миеломы. Также было показано, что он задерживает повторное появление многочисленной миеломы после лечения.
Миелодиспластические синдромы (МДС)
МДС - это группа различных заболеваний крови и костного мозга. Кровяные клетки становятся аномальными и не функционируют правильно. Пациенты могут испытывать различные симптомы, включая низкий уровень красных кровяных клеток (анемию), необходимость переливания крови и риск инфекции.
Леналидомида используется для лечения пациентов с МДС, когда все следующие условия применяются:
- пациент нуждается в периодических переливаниях крови для лечения низкого уровня красных кровяных клеток ("анемия, зависимая от переливания").
- пациент имеет аномалию клеток костного мозга, называемую "изолированной делецией 5q". Это означает, что организм пациента не производит достаточного количества здоровых кровяных клеток.
- другие методы лечения, которые пациент использовал ранее, не являются подходящими или не работают достаточно хорошо.
Леналидомида может увеличить количество здоровых красных кровяных клеток, производимых организмом, уменьшая количество аномальных клеток:
- это может уменьшить количество переливаний крови, необходимых пациенту. Возможно, переливания крови не будут необходимы.
Лимфома мантийных клеток (ЛМК)
ЛМК - это рак одной части иммунной системы (лимфатической ткани). Он поражает определенный тип белых кровяных клеток, называемых "лимфоцитами B" или клетками B. ЛМК - это заболевание, при котором клетки B растут без контроля и накапливаются в лимфатической ткани, костном мозге или крови.
Леналидомида используется в качестве монотерапии для лечения пациентов, которые ранее получали лечение другими лекарствами.
Фолликулярный лимфома (ФЛ)
ФЛ - это медленно растущий рак, который поражает лимфоциты B. Это тип белых кровяных клеток, которые помогают организму бороться с инфекциями. Когда человек страдает ФЛ, он может накапливать слишком много этих лимфоцитов B в крови, костном мозге, лимфатических узлах и селезенке.
Леналидомида используется вместе с другим лекарством под названием "ритуксимаб" для лечения пациентов, которые ранее получали лечение фолликулярным лимфомой.
Как действует Леналидомида Ауровитас
Леналидомида действует, влияя на иммунную систему организма и trực tiếp атакуя рак. Он действует разными способами:
- останавливает развитие раковых клеток.
- останавливает рост кровеносных сосудов в раке.
- стимулирует часть иммунной системы для атаки на раковые клетки.
2. Что нужно знать перед началом приема Леналидомида Ауровитас
Прежде чем начать лечение леналидомидой, прочитайте прошпект всех лекарств, которые будут использоваться в комбинации с леналидомидой.
Не принимайте Леналидомида Ауровитас
- если вы беременны, думаете, что можете быть беременной или планируете стать беременной, поскольку ожидается, что леналидомида будет вредна для плода (см. раздел 2, "Беременность, лактация и контрацепция: информация для женщин и мужчин").
- если вы можете стать беременной, если не будут приняты все необходимые меры для предотвращения этого (см. раздел 2, "Беременность, лактация и контрацепция: информация для женщин и мужчин"). Если вы можете стать беременной, ваш врач запишет с каждым рецептом, что были приняты все необходимые меры, и предоставит вам это подтверждение.
- если вы аллергичны к леналидомиде или любому другому компоненту этого лекарства (перечисленному в разделе 6). Если вы думаете, что можете быть аллергичны, проконсультируйтесь с вашим врачом.
Если любое из этих условий применяется к вам, не принимайте леналидомида. В случае сомнений проконсультируйтесь с вашим врачом.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом, фармацевтом или медсестрой перед началом приема Леналидомида Ауровитас, если:
- у вас когда-либо были тромбы крови; во время лечения, у вас повышается риск развития тромбов крови в венах и артериях.
- у вас есть какие-либо признаки инфекции, такие как кашель или температура.
- у вас есть или ранее была вирусная инфекция, особенно инфекция вируса гепатита B, ветряной оспы или ВИЧ. В случае сомнений проконсультируйтесь с вашим врачом. Лечение леналидомидой может привести к реактивации вируса у пациентов, которые являются носителями вируса. Это приводит к повторному появлению инфекции. Ваш врач должен проверить, имели ли вы когда-либо инфекцию вируса гепатита B.
- у вас есть проблемы с почками; ваш врач может скорректировать дозу леналидомиды.
- у вас был инфаркт миокарда, у вас когда-либо был тромб крови, или если вы курите, у вас высокое кровяное давление или высокий уровень холестерина.
- у вас была аллергическая реакция при использовании талидомиды (другого лекарства, используемого для лечения многочисленной миеломы), такой как кожная сыпь, зуд, отек, головокружение или проблемы с дыханием.
- у вас была в прошлом комбинация любых из следующих симптомов: общая кожная сыпь, покраснение кожи, высокая температура, симптомы, подобные гриппу, повышение уровня печеночных ферментов, аномалии крови (эозинофилия), увеличенные лимфатические узлы (это признаки тяжелой кожной реакции, называемой реакцией на лекарство с эозинофилией и системными симптомами, также известной как синдром DRESS или синдром гиперчувствительности к лекарству) (см. также раздел 4 "Возможные побочные эффекты").
Если любое из этих условий применяется к вам, сообщите об этом вашему врачу, фармацевту или медсестре перед началом лечения.
В любое время, во время или после лечения, сообщите вашему врачу или медсестре немедленно, если у вас:
- размытое зрение, потеря зрения или двойное зрение, трудности с речью, слабость в руке или ноге, изменение походки или проблемы с равновесием, онемение, уменьшение чувствительности или потеря чувствительности, потеря памяти или путаница. Все эти симптомы могут быть признаками тяжелого и потенциально смертельного заболевания мозга, называемого прогрессивной мультифокальной лейкоэнцефалопатией (ПМЛ). Если у вас есть любой из этих симптомов перед началом лечения леналидомидой, сообщите вашему врачу, если вы заметите какие-либо изменения в этих симптомах.
- затруднение дыхания, усталость, головокружение, боль в груди, более быстрый сердечный ритм или отек в ногах или лодыжках. Это могут быть симптомы тяжелого состояния, называемого легочной гипертензией (см. раздел 4).
Анализы и тесты
Перед началом лечения леналидомидой и во время лечения у вас будут регулярно проводиться анализы крови. Это связано с тем, что леналидомида может вызвать уменьшение количества кровяных клеток, помогающих бороться с инфекциями (белых кровяных клеток) и участвующих в свертывании крови (тромбоцитов).
Ваш врач попросит вас сделать анализ крови:
- перед началом лечения.
- каждую неделю в течение первых 8 недель лечения.
- затем не менее чем один раз в месяц.
Возможно, вам будет предложено пройти обследование для выявления признаков сердечно-легочных проблем перед и во время лечения леналидомидой.
Для пациентов с МДС, принимающих леналидомида
Если у вас МДС, вы можете быть более склонны к развитию более тяжелого заболевания, называемого острой миелоидной лейкемией (ОМЛ). Кроме того, неизвестно, как леналидомида влияет на вероятность развития ОМЛ. Ваш врач, поэтому, может назначить вам анализы для выявления признаков, которые могут предсказать лучшую вероятность развития ОМЛ во время лечения леналидомидой.
Для пациентов с ЛМК, принимающих леналидомида
Ваш врач попросит вас сделать анализ крови:
- перед началом лечения
- каждую неделю в течение первых 8 недель (2 цикла) лечения
- затем каждые 2 недели в циклах 3 и 4 (см. раздел 3 "Цикл лечения" для получения более подробной информации)
- после этого, анализ будет проводиться в начале каждого цикла и
- не менее чем один раз в месяц
Для пациентов с ФЛ, принимающих леналидомида
Ваш врач попросит вас сделать анализ крови:
- перед началом лечения
- каждую неделю в течение первых 3 недель (1 цикл) лечения
- затем каждые 2 недели в циклах 2-4 (см. раздел 3 "Цикл лечения" для получения более подробной информации)
- после этого, анализ будет проводиться в начале каждого цикла и
- не менее чем один раз в месяц
Ваш врач может проверить, есть ли у вас высокий уровень опухоли в организме, включая костный мозг. Это может привести к развитию заболевания, при котором опухоли разрушаются и производят необычные количества химических веществ в крови, что может привести к почечной недостаточности (это заболевание называется "синдром лизиса опухоли").
Ваш врач может осмотреть вас для выявления изменений в коже, таких как красные пятна или кожная сыпь.
Ваш врач может скорректировать дозу леналидомиды или прекратить лечение, в зависимости от результатов анализов крови и общего состояния. Если вы пациент с новым диагнозом, ваш врач может также оценить ваше лечение в зависимости от возраста и других заболеваний, которые у вас уже есть.
Донорство крови
Вы не должны жертвовать кровь во время лечения и не менее чем 7 дней после окончания лечения.
Дети и подростки
Не рекомендуется использовать леналидомида у детей и подростков младше 18 лет.
Пациенты пожилого возраста и лица с проблемами почек
Если вам 75 лет или более, или у вас есть проблемы с почками средней или тяжелой степени, ваш врач будет внимательно следить за вами перед началом лечения.
Другие лекарства и Леналидомида Ауровитас
Сообщите вашему врачу или медсестре, если вы принимаете, недавно принимали или можете принимать любое другое лекарство. Это связано с тем, что леналидомида может влиять на то, как работают другие лекарства. Кроме того, некоторые лекарства могут влиять на то, как работает леналидомида.
В частности, сообщите вашему врачу или медсестре, если вы принимаете любое из следующих лекарств:
- некоторые лекарства, используемые для предотвращения беременности, такие как оральные контрацептивы, поскольку они могут перестать работать.
- некоторые лекарства, используемые для проблем с сердцем, такие как дигоксин.
- некоторые лекарства, используемые для разжижения крови, такие как варфарин.
Беременность, лактация и контрацепция: информация для женщин и мужчин
Беременность
Женщины, принимающие леналидомида
- Не принимайте леналидомида, если вы беременны, поскольку ожидается, что она будет вредна для плода.
- Не становитесь беременными во время приема леналидомиды. Поэтому вам необходимо использовать эффективные методы контрацепции, если существует вероятность того, что вы можете стать беременной (см. "Контрацепция").
- Если вы становитесь беременной во время лечения леналидомидой, прекратите лечение и немедленно сообщите об этом вашему врачу.
Мужчины, принимающие леналидомида
- Если ваша партнерша становится беременной во время приема леналидомиды, немедленно сообщите об этом вашему врачу. Рекомендуется, чтобы ваша партнерша обратилась за медицинской консультацией.
- Вы также должны использовать эффективные методы контрацепции (см. "Контрацепция").
Лактация
Не кормите грудью во время приема леналидомиды, поскольку неизвестно, проникает ли леналидомида в грудное молоко.
Контрацепция
Для женщин, принимающих леналидомида
Перед началом лечения спросите вашего врача, можете ли вы стать беременной, даже если вы думаете, что это маловероятно.
Если вы можете стать беременной:
- вам будут проводиться тесты на беременность под медицинским контролем (перед каждым лечением, не менее чем каждые 4 недели во время лечения и не менее чем 4 недели после окончания лечения) за исключением случаев, когда подтверждено, что у вас сделана тубальная лигация, чтобы яйцеклетки не достигали матки (тубальная лигация).
И
- вам необходимо использовать эффективные методы контрацепции не менее чем за 4 недели до начала лечения, во время лечения и не менее чем 4 недели после окончания лечения. Ваш врач посоветует вам наиболее подходящие методы контрацепции.
Для мужчин, принимающих леналидомида
Леналидомида проникает в человеческую сперму. Если ваша партнерша беременна или может стать беременной и не использует эффективные методы контрацепции, вы должны использовать презервативы во время лечения и не менее чем 7 дней после окончания лечения, даже если вы прошли вазэктомию. Не жертвуйте сперму или семя во время лечения и не менее чем 7 дней после окончания лечения.
Вождение и использование машин
Не驾айте транспортные средства и не используйте машины, если вы чувствуете головокружение, усталость, сонливость, имеете проблемы с зрением или равновесием после приема леналидомиды.
Леналидомида Ауровитас содержит лактозу
Это лекарство содержит лактозу. Если ваш врач сказал вам, что у вас есть непереносимость некоторых сахаров, проконсультируйтесь с ним перед приемом этого лекарства.
Леналидомида Ауровитас содержит натрий
Это лекарство содержит менее 1 ммоль натрия (23 мг) на капсулу; это означает, что оно практически "не содержит натрия".
3. Как принимать Леналидомид Ауровитас
Леналидомид должен быть назначен медицинским специалистом с опытом лечения множественной миеломы, СМД, ЛКМ или ЛФ.
- Когда леналидомид используется для лечения множественной миеломы у пациентов, которые не могут быть лечены трансплантацией костного мозга или прошли другие методы лечения ранее, его принимают вместе с другими препаратами (см. раздел 1 «Для чего используется Леналидомид Ауровитас»).
- Когда леналидомид используется для лечения множественной миеломы у пациентов, которые прошли трансплантацию костного мозга или для лечения пациентов с СМД или ЛКМ, его принимают отдельно.
- Когда леналидомид используется для лечения фолликулярного лимфомы, его принимают вместе с другим препаратом под названием «ритуксимаб».
Следуйте точно инструкциям по приему этого препарата, указанным вашим врачом. В случае сомнений проконсультируйтесь снова с вашим врачом или фармацевтом.
Если вы принимаете леналидомид вместе с другими препаратами, вам следует проконсультироваться с инструкциями этих других препаратов, чтобы получить дополнительную информацию о их использовании и эффектах.
Цикл лечения
Леналидомид принимается в определенные дни в течение периода 3 недель (21 день).
- «Цикл лечения» состоит из 21 дня.
- В зависимости от дня цикла, вы будете принимать один или несколько препаратов. Однако в некоторые дни вы не будете принимать никаких препаратов.
- После завершения каждого цикла из 21 дня, вам необходимо начать новый «цикл» в течение следующих 21 дней.
Или
Леналидомид принимается в определенные дни в течение периода 4 недель (28 дней).
- «Цикл лечения» состоит из 28 дней.
- В зависимости от дня цикла, вы будете принимать один или несколько препаратов. Однако в некоторые дни вы не будете принимать никаких препаратов.
- После завершения каждого цикла из 28 дней, вам необходимо начать новый «цикл» в течение следующих 28 дней.
Сколько принимать Леналидомид Ауровитас
До начала лечения, ваш врач укажет вам:
- какое количество леналидомида вам необходимо принимать.
- какое количество других препаратов вам необходимо принимать вместе с леналидомидом, если это необходимо.
- в какие дни цикла лечения вам необходимо принимать каждый препарат.
Как и когда принимать Леналидомид Ауровитас
- Проглатывайте капсулы целиком, предпочтительно с водой.
- Не разламывайте, не открывайте и не жуйте капсулы. В случае, если порошок разбитой капсулы леналидомида соприкасается с кожей, немедленно и тщательно промойте кожу водой и мылом.
- Медицинские специалисты, ухаживающие лица и члены семьи должны использовать одноразовые перчатки при обращении с блистером или капсулой. После этого, перчатки необходимо осторожно снять, чтобы избежать кожного контакта, положить в полиэтиленовый пакет и утилизировать в соответствии с местными требованиями. Затем, необходимо тщательно промыть руки водой и мылом. Беременные женщины или те, кто подозревает, что могут быть беременными, не должны обращаться с блистером или капсулой.
- Капсулы можно принимать с пищей или без нее.
- Вы должны принимать леналидомид примерно в одно и то же время в назначенные дни.
Прием этого препарата
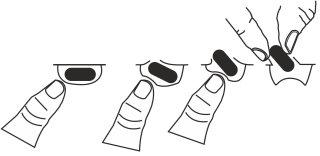
Чтобы вынуть капсулу из блистера:
- Нажмите только на один конец капсулы, чтобы она вышла через ламинат.
- Не нажимайте на центр капсулы, так как это может导致 ее разрушения.
Продолжительность лечения Леналидомидом Ауровитас
Леналидомид принимается в циклах лечения, каждый цикл длится 21 или 28 дней (см. «Цикл лечения» выше). Вы должны продолжать циклы лечения до тех пор, пока ваш врач не укажет вам прекратить лечение.
Если вы приняли больше Леналидомида Ауровитас, чем необходимо
Если вы приняли больше леналидомида, чем вам было назначено, немедленно сообщите об этом вашему врачу.
В случае передозировки или случайного приема, немедленно проконсультируйтесь с вашим врачом или фармацевтом или позвоните в Службу токсикологической информации, телефон 91 562 04 20, указав препарат и количество, принятое внутрь.
Если вы забыли принять Леналидомид Ауровитас
Если вы забыли принять леналидомид в назначенное время и:
- прошло менее 12 часов: принимайте капсулу немедленно.
- прошло более 12 часов: не принимайте капсулу. Принимайте следующую капсулу на следующий день в назначенное время.
Если у вас есть какие-либо другие вопросы о использовании этого препарата, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все препараты, этот препарат может вызывать побочные эффекты, хотя не все люди испытывают их.
Если вы испытываете любой из следующих серьезных побочных эффектов, прекратите лечение леналидомидом и немедленно обратитесь к врачу, поскольку может потребоваться срочная медицинская помощь:
- Крапивница, высыпания, отек глаз, рта или лица, затруднение дыхания или зуд, которые могут быть симптомами тяжелых аллергических реакций, называемых ангиеодемой и анафилактической реакцией.
- Тяжелая аллергическая реакция, которая может начаться как высыпание в одной области, но распространяется, вызывая значительную потерю кожи по всему телу (синдром Стивенса-Джонсона и/или токсический эпидермальный некролиз).
- Общая высыпание, высокая температура, увеличение печеночных ферментов, аномалии в крови (эозинофилия), увеличенные лимфатические узлы и эффекты на другие органы тела (реакция на препарат с эозинофилией и системными симптомами, также известная как синдром DRESS или синдром гиперчувствительности к препарату). См. также раздел 2.
Немедленно проконсультируйтесь с вашим врачом, если вы заметите любой из следующих серьезных побочных эффектов:
- Лихорадка, озноб, боль в горле, кашель, язвы во рту или любой другой симптом инфекции, включая инфекцию крови (сепсис).
- Кровотечение (кровотечение) или гематома (синяк) не связанные с травмой.
- Боль в груди (торакальная) или в ногах.
- Затруднение дыхания.
- Боль в костях, боль в мышцах, спутанность сознания или усталость, которые могут быть вызваны высокими уровнями кальция в крови.
Леналидомид может снижать количество белых кровяных клеток, которые борются с инфекциями, и также количество клеток крови, которые помогают свертывать кровь (тромбоциты), что может привести к кровотечениям, таким как носовые кровотечения и синяки. Леналидомид также может вызывать тромбы в венах (тромбоз).
Другие побочные эффекты
Важно отметить, что небольшое количество пациентов может развить другие виды рака, и возможно, что этот риск увеличивается с лечением леналидомидом. Поэтому, ваш врач должен тщательно оценить преимущества и риски при назначении леналидомида.
Побочные эффекты очень часто(могут поражать более 1 из 10 человек):
- Снижение количества красных кровяных клеток, что может привести к анемии, вызывающей усталость и слабость.
- Высыпание на коже, зуд.
- Мышечные спазмы, мышечная слабость, боль в мышцах, дискомфорт в мышцах, боль в костях, боль в суставах, боль в спине, боль в конечностях.
- Общая отечность, включая отечность рук и ног.
- Слабость, усталость.
- Лихорадка и псевдогриппозные симптомы, включая лихорадку, боль в мышцах, боль в голове, боль в ушах, кашель и озноб.
- Онемение, покалывание или неприятное ощущение в коже, боль в руках или ногах, головокружение, тремор.
- Снижение аппетита, изменения во вкусе.
- Увеличение боли, размера опухоли или покраснения вокруг опухоли.
- Потеря веса.
- Запор, диарея, тошнота, рвота, боль в животе, изжога.
- Низкие уровни калия или кальция и/или натрия в крови.
- Снижение функции щитовидной железы.
- Боль в ногах (которая может быть симптомом тромбоза), боль в груди или затруднение дыхания (которые могут быть симптомами тромбов в легких, называемых легочной эмболией).
- Инфекции любого вида, включая инфекцию пазух носа (синусит), инфекцию легких и верхних дыхательных путей.
- Затруднение дыхания.
- Размытое зрение.
- Помутнение глаза (катаракта).
- Проблемы с почками, включая почки, которые не функционируют правильно или не могут поддерживать нормальную функцию.
- Аномальные результаты печеночных тестов.
- Высокие уровни печеночных ферментов.
- Изменения в белке крови, которые могут привести к отеку артерий (васкулит).
- Увеличение уровня сахара в крови (диабет).
- Снижение уровня сахара в крови.
- Боль в голове.
- Носовые кровотечения.
- Сухая кожа.
- Депрессия, изменения в настроении, трудности со сном.
- Кашель.
- Снижение артериального давления.
- Размытое чувство дискомфорта в теле, чувство недомогания.
- Больное воспаление рта, сухость во рту.
- Обезвоживание.
Побочные эффекты часто(могут поражать до 1 из 10 человек):
- Разрушение красных кровяных клеток (гемолитическая анемия).
- Определенные виды кожных опухолей.
- Кровотечение из десен, желудка или кишечника.
- Увеличение артериального давления, медленный, быстрый или нерегулярный сердечный ритм.
- Увеличение количества вещества, которое высвобождается после нормального или аномального разрушения красных кровяных клеток.
- Увеличение типа белка, который указывает на воспаление в организме.
- Затемнение цвета кожи; изменение цвета кожи в результате внутреннего кровотечения, обычно вызванного синяками; воспаление кожи, вызванное накоплением крови; гематома.
- Увеличение уровня мочевой кислоты в крови.
- Кожные высыпания, покраснение кожи, трещины кожи, отслоение или шелушение кожи, крапивница.
- Увеличение потливости, ночная потливость.
- Затруднение глотания, боль в горле, трудности с поддержанием качества голоса или изменения в голосе.
- Насморк.
- Сильное увеличение или уменьшение количества мочи по сравнению с обычным или невозможность контролировать мочеиспускание.
- Кровь в моче.
- Затруднение дыхания, особенно при лежании (которое может быть симптомом сердечной недостаточности).
- Трудности с эрекцией.
- Инсульт, обморок, головокружение (расстройство внутреннего уха, вызывающее чувство, что все кружится), временная потеря сознания.
- Боль в груди, которая распространяется на руки, шею, челюсть, спину или живот, чувство потливости и нехватки воздуха, тошнота или рвота, которые могут быть симптомами сердечного приступа (инфаркта миокарда).
- Мышечная слабость, отсутствие энергии.
- Боль в шее, боль в груди.
- Озноб.
- Отек суставов.
- Замедление или блокировка желчи в печени.
- Низкие уровни фосфата или магния в крови.
- Трудности с речью.
- Повреждение печени.
- Нарушение равновесия, трудности с движениями.
- Глухота, звон в ушах (тиннитус).
- Боль в нервах, необычное и неприятное ощущение, особенно при прикосновении.
- Избыток железа в организме.
- Жажда.
- Спутанность сознания.
- Боль в зубах.
- Падение, которое может привести к травмам.
Побочные эффекты редко(могут поражать до 1 из 100 человек):
- Кровотечение внутри черепа.
- Проблемы с кровообращением.
- Потеря зрения.
- Потеря полового влечения (либидо).
- Выделение большого количества мочи с болью в костях и слабостью, которые могут быть симптомами почечного расстройства (синдром Фанкони).
- Желтушная окраска кожи, слизистых оболочек или глаз (желтуха), бледный стул, темная моча, зуд кожи, высыпание, боль или отек живота; эти могут быть симптомами повреждения печени (печеночной недостаточности).
- Боль в животе, отек живота или диарея, которые могут быть симптомами воспаления толстой кишки (колита или тифлита).
- Повреждение клеток почек (называемое тубулярной некрозом почек).
- Изменения цвета кожи, чувствительность к солнечному свету.
- Синдром лизиса опухоли – могут возникать метаболические осложнения во время лечения рака и иногда даже без лечения. Эти осложнения возникают в результате продуктов распада опухолевых клеток, которые умирают, и могут включать: изменения в биохимии крови, высокие уровни калия, фосфора, мочевой кислоты и низкие уровни кальция, которые, в свою очередь, приводят к изменениям в функции почек и сердечном ритме, судорогам и, иногда, смерти.
- Увеличение артериального давления внутри кровеносных сосудов, которые снабжают легкие (легочная гипертензия).
Побочные эффекты частота неизвестна(не может быть оценена из доступных данных):
- Внезапная боль или легкая боль в верхней части живота и/или спине, которая длится несколько дней, возможно, сопровождаемая тошнотой, рвотой, лихорадкой и быстрым сердечным ритмом. Эти симптомы могут быть вызваны воспалением поджелудочной железы.
- Свист или звон при дыхании, затруднение дыхания или сухой кашель, которые могут быть симптомами, вызванными воспалением легочной ткани.
- Были зарегистрированы редкие случаи разрушения мышц (боль, слабость или отек мышц), которые могут привести к проблемам с почками (рабдомиолиз), некоторые из которых возникают при приеме леналидомида с статином (типом препарата для снижения уровня холестерина).
- Заболевание, поражающее кожу, вызванное воспалением мелких кровеносных сосудов, сопровождаемое болью в суставах и лихорадкой (васкулит леукоциклостаз).
- Разрыв стенки желудка или кишечника. Это может привести к очень серьезной инфекции. Сообщите вашему врачу, если у вас есть сильная боль в животе, лихорадка, тошнота, рвота, кровь в стуле или изменения в кишечных привычках.
- Вирусные инфекции, включая герпес зостер (также известный как «поясничный герпес», вирусное заболевание, которое вызывает болезненную кожную сыпь с пузырьками) и повторное возникновение инфекции вируса гепатита Б (которое может привести к желтушной окраске кожи и глаз, темной моче, боли в правом верхнем квадранте живота, лихорадке и тошноте или чувству недомогания).
- Отказ от трансплантации твердых органов (например, почки, сердца).
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не перечислены в этом листке. Вы также можете сообщить об этом напрямую через Испанскую систему фармакологического надзора за лекарствами для человека: www.notificaram.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого препарата.
5. Хранение Леналидомида Ауровитас
Храните этот препарат вне поля зрения и досягаемости детей.
Не используйте этот препарат после даты истечения срока годности, указанной на упаковке и блистере, после «CAD». Дата истечения срока годности – последний день месяца, указанного.
Храните при температуре ниже 30°C.
Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в пункт сбора SIGRE в аптеке. Спросите вашего фармацевта, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
СоставЛеналидомида Ауровитас
- Активное вещество - леналидомид.
Каждая твердая капсула содержит 5 мг леналидомида.
Каждая твердая капсула содержит 10 мг леналидомида.
Каждая твердая капсула содержит 15 мг леналидомида.
Каждая твердая капсула содержит 20 мг леналидомида.
Каждая твердая капсула содержит 25 мг леналидомида.
- Другие компоненты:
Содержание капсулы:лактоза, микрокристаллическая целлюлоза (град 102), натрий кроскармеллоза и магний стеарат.
Покрытие капсулы:диоксид титана (E171), желтый оксид железа (E172) (только для 10 мг и 20 мг), кармин индиго (E132) (только для 10 мг), красный оксид железа (E172) (только для 10 мг, 15 мг и 20 мг) и желатина.
Печатная краска:лака Шеллака, черный оксид железа (E172), гидроксид калия.
Внешний вид продукта и содержание упаковки
Леналидомид Ауровитас 5 мг твердые капсулы EFG[размер примерно 17,8 мм]
Твердая желатиновая капсула размера "2", с крышкой белого цвета и телом белого цвета, с надписью "L5" черной краской, заполненная белым или светло-желтым порошком.
Леналидомид Ауровитас 10 мг твердые капсулы EFG[размер примерно 21,4 мм]
Твердая желатиновая капсула размера "0", с крышкой оливкового цвета и телом оранжевого цвета, с надписью "L10" черной краской, заполненная белым или светло-желтым порошком.
Леналидомид Ауровитас 15 мг твердые капсулы EFG[размер примерно 21,4 мм]
Твердая желатиновая капсула размера "0", с крышкой темно-оранжевого цвета и телом темно-оранжевого цвета, с надписью "L15" черной краской, заполненная белым или светло-желтым порошком.
Леналидомид Ауровитас 20 мг твердые капсулы EFG[размер примерно 21,4 мм]
Твердая желатиновая капсула размера "0", с крышкой оранжевого цвета и телом оранжевого цвета, с надписью "L20" черной краской, заполненная белым или светло-желтым порошком.
Леналидомид Ауровитас 25 мг твердые капсулы EFG[размер примерно 21,4 мм]
Твердая желатиновая капсула размера "0", с крышкой белого цвета и телом белого цвета, с надписью "L25" черной краской, заполненная белым или светло-желтым порошком.
Размеры упаковки:
Блистер:7, 14, 21, 28 и 42 твердых капсулы.
Возможно, не все размеры упаковки будут продаваться.
Владелец разрешения на маркетинг и ответственный за производство
Владелец разрешения на маркетинг
Eugia Pharma (Malta) Limited
Vault 14, Level 2, Valletta Waterfront
Floriana, FRN 1914
Мальта
Ответственный за производство
APL Swift Services (Malta) Limited
HF26, Hal Far Industrial Estate, Hal Far
Birzebbugia, BBG 3000
Мальта
Или
Generis Farmacêutica, S.A.
Rua João de Deus, 19
2700-487 Amadora
Португалия
Или
Arrow Génériques
26 Avenue Tony Garnier
69007 Lyon
Франция
Для получения дополнительной информации о этом лекарственном средстве можно обратиться к местному представителю владельца разрешения на маркетинг:
Aurovitas Spain, S.A.U.
Avda. de Burgos, 16-D
28036 Madrid
Испания
Это лекарственное средство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Германия: Lenalidomid PUREN 2,5 мг/5 мг/7,5 мг/10 мг/15 мг/20 мг/25 мг твердые капсулы
Бельгия: Lenalidomide AB 2.5 мг/5 мг/7.5 мг/10 мг/15 мг/20 мг/25 мг, желатиновые капсулы, твердые капсулы
Испания: Lenalidomida Aurovitas 5 мг/10 мг/15 мг/20 мг/25 мг твердые капсулы EFG
Франция: Lenalidomide Arrow 2.5 мг, 5 мг, 7.5 мг, 10 мг, 15 мг, 20 мг и 25 мг, желатиновые капсулы
Нидерланды: Lenalidomide Eugia 2,5 мг, 5 мг, 7,5 мг, 10 мг, 15 мг, 20 мг, 25 мг, твердые капсулы
Польша: Lenalidomide Eugia
Португалия: Lenalidomida Generis
Дата последнего пересмотра этого листка-вкладыша:июль 2023 г.
Подробная информация о этом лекарственном средстве доступна на сайте Агентства по лекарственным средствам и медицинским изделиям Испании (AEMPS) (http://www.aemps.gob.es/).
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ЛЕНАЛИДОМИД Ауровитас 15 мг ТВЕРДЫЕ КАПСУЛЫФорма выпуска: КАПСУЛА, 10 мгАктивное вещество: Леналидомид.Производитель: Accord Healthcare S.L.U.Требуется рецептФорма выпуска: КАПСУЛА, 15 мгАктивное вещество: Леналидомид.Производитель: Accord Healthcare S.L.U.Требуется рецептФорма выпуска: КАПСУЛА, 2,5 мгАктивное вещество: Леналидомид.Производитель: Accord Healthcare S.L.U.Требуется рецепт
Аналоги ЛЕНАЛИДОМИД Ауровитас 15 мг ТВЕРДЫЕ КАПСУЛЫ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ЛЕНАЛИДОМИД Ауровитас 15 мг ТВЕРДЫЕ КАПСУЛЫ в Польша
Аналог ЛЕНАЛИДОМИД Ауровитас 15 мг ТВЕРДЫЕ КАПСУЛЫ в Украина
Врачи онлайн по ЛЕНАЛИДОМИД Ауровитас 15 мг ТВЕРДЫЕ КАПСУЛЫ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ЛЕНАЛИДОМИД Ауровитас 15 мг ТВЕРДЫЕ КАПСУЛЫ – по решению врача и с учетом местных правил.






