HUMATROPE 24 mg POWDER AND SOLVENT FOR INJECTION


How to use HUMATROPE 24 mg POWDER AND SOLVENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Humatrope 6 mg/ 12 mg/ 24 mg powder and solvent for solution for injection
somatropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Humatrope and what is it used for
- What you need to know before you use Humatrope
- How to use Humatrope
- Possible side effects
- Storing Humatrope
- Contents of the pack and other information
1. What is Humatrope and what is it used for
Your medicine or the medicine of the person in your care is called Humatrope. It contains human growth hormone, also called somatropin. Humatrope is made by a special process called recombinant DNA technology. It has the same structure as the growth hormone that your body produces.
Growth hormone regulates the growth and development of the cells in your body. When it stimulates the growth of cells in the spine and in the long bones of the legs, it causes an increase in height.
In cases of growth hormone deficiency, growth hormone also increases bone mineral content, the number and size of muscle cells, and reduces body fat deposits.
Humatrope is used for
- Treatment of children and adolescents with any of the following growth disorders:
- Insufficient production of growth hormone (growth hormone deficiency),
- Absence of all or some X sex chromosomes in short-statured females (Turner Syndrome),
- A disease in which the kidneys are damaged (chronic kidney disease) in children with growth retardation before puberty,
- Short stature at birth (SGA = small for gestational age) that has not caught up by 4 years of age or later,
- A genetic disorder called SHOX deficiency.
- Treatment of adults who have confirmed growth hormone deficiency starting either in childhood or in adulthood.
2. What you need to know before you use Humatrope
Do not use Humatrope
- if you are allergic(hypersensitive) to somatropin or any of the other ingredients of Humatrope (e.g. metacresol, glycerol of the solvent) see section 6.
- and inform your doctor if you have an active tumor (cancer). Tumors must be inactive and you must have finished anti-tumor treatment before starting treatment with Humatrope.
- if you have stopped growingand want to increase growth in height (growth plates at the end of the long bones closed). Your doctor will examine you and decide if you still need Humatrope after your bones have stopped growing.
- if you are very illand require intensive care due to a serious heart or abdominal intervention, or have been treated for multiple injuries after an accident, or require treatment to help you breathe after suffering from acute respiratory failure.
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Humatrope.
If you are receiving glucocorticoid replacement therapy, you should consult your doctor regularly as your glucocorticoid dose may need to be adjusted.
If you were treated for growth hormone deficiency during childhood, your doctor will re-examine your growth hormone deficiency to decide if you need treatment with Humatrope during adulthood.
If you have completed previous anti-tumor treatment, a brain scan may be necessary before starting treatment with Humatrope. You should be examined regularly to ensure that the tumor does not come back or start growing again.
There has been a reported increased risk of developing a second tumor (benign or malignant) in patients who survived cancer and were treated with somatropin. Of these second tumors, brain tumors were the most common.
If you have symptoms such as frequent or severe headaches with nausea and/or vision problems, inform your doctor immediately. Your doctor should perform an eye examination to see if there is evidence of increased pressure in the brain. Depending on the results of this examination, treatment with Humatrope may need to be interrupted.
If you experience limping or hip pain, please consult your doctor. During growth periods, changes in the hip bone can occur.
If you start treatment, Humatrope may affect the amount of thyroid hormones in your blood. If your thyroid hormone level is low, it may reduce your response to Humatrope. Therefore, you should have regular thyroid function tests, regardless of whether you are receiving thyroid hormone treatment or not.
If you are a child, make sure to continue treatment until you reach your final height.
If you take a higher dose than prescribed for Humatrope, you may experience overgrowth of some parts of your body such as ears, nose, jaw, hands, and feet. Overdose can also cause an increase in blood sugar levels and sugar in the urine. Always use Humatrope as your doctor has indicated.
If you have had growth disorders due to kidney damage, you should stop treatment with Humatrope before kidney transplantation.
If you have critical illnesses, you should inform the doctor treating you. There have been reports of death in patients who were receiving somatropin during critical illnesses.
If you have growth hormone deficiency and also have Prader-Willi syndrome (a genetic disorder), your doctor should examine your respiratory problems and airway infections before starting treatment with Humatrope, especially if you are overweight, have had severe respiratory problems in the past (especially during sleep), or have suffered from lung or airway infections. If during treatment you have signs of respiratory problems (snoring), treatment should be interrupted and the cause evaluated by your doctor.
Humatrope may affect how your body acts on the sugar from food and drink, interfering with how your body uses insulin. Therefore, if you take Humatrope, your doctor should confirm if your body is handling sugar correctly.
If you have diabetes mellitus, you may need an adjustment in your insulin dose after starting treatment with Humatrope. Your doctor will check your blood sugar levels and may adjust your diabetes treatment.
If you have a growth disorder associated with being born small for gestational age, your blood sugar and insulin levels should be measured before starting treatment and regularly during treatment.
If you are an elderly patient (over 65 years), you may be more sensitive to Humatrope and may be prone to side effects.
Humatrope may cause pancreatitis, which causes severe abdominal and back pain. Contact your doctor if you or your child develops stomach pain after taking Humatrope.
Scoliosis (an increase in the lateral curvature of the spine) may progress in any child during rapid growth. Signs of scoliosis should be monitored during treatment.
Other medicines and Humatrope
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
In particular, tell your doctor if you are taking or have recently taken any of the following medicines. Your doctor may need to adjust the dose of Humatrope or the other medicines:
- medicines for the treatment of diabetes mellitusthat may need to be adjusted.
- adrenal steroid hormone(glucocorticoid), such as cortisone or prednisolone; your doctor may need to adjust the dose because the combination of these medicines with Humatrope may reduce the effect of both treatments.
- estrogens administered orally or other sex hormones, as they may affect the response to growth hormone treatment. If there is a change in how estrogens are taken (e.g. from oral to transdermal: through the skin); it may be necessary to adjust the dose of Humatrope.
- medicines to prevent seizures (anticonvulsants) or cyclosporin.
Pregnancy and breastfeeding
Humatrope should not be used during pregnancy, unless your doctor tells you to. Inform your doctor immediately if you are pregnant.
It is not known if somatropin passes into breast milk. If you are breastfeeding or plan to breastfeed, consult your doctor before using Humatrope.
Driving and using machines
Humatrope has no known effects on the ability to drive or use machines.
Humatrope contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per daily dose; this is, essentially sodium-free.
Use in athletes
This medicine contains somatropin which may produce a positive result in doping tests.
3. How to use Humatrope
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are not sure, consult your doctor or pharmacist again.
- Always make sure to use the cartridge with the concentration that your doctor has prescribed (concentration of 6 mg, 12 mg or 24 mg) and the correct Humatrope pen injection system with CE marking. Never use cartridges of other medicines in your Humatrope pen.
- Each Humatrope cartridge comes with a syringe that contains a diluent (solvent for solution for injection) for its reconstitution (mixing and preparation of the solution for injection).
- Do not mix or inject Humatrope until you have received sufficient training from your doctor or other qualified healthcare professionals.
- For detailed instructions on how to prepare and inject Humatrope, see the section “How to inject Humatrope”at the end of this leaflet. You should only mix Humatrope with the provided diluent. Never mix it with anything else unless your doctor tells you to.
- After reconstitution, Humatrope should be injected into the fatty tissue just under the skin using a short needle and a pen injection system.
- The injection sites should be varied to avoid reduction and hardening of the fatty tissue under the skin (lipoatrophy).
- After mixing Humatrope, do not leave it out of the refrigerator for more than 30 minutes per day.
- Keep your pen with the remaining Humatrope in the refrigerator. Do not use leftover Humatrope after 28 days after mixing.
Dose
Your doctor will tell you what dose and administration schedule to use. Do not change your dose without consulting your doctor.
Normally, treatment with Humatrope is long-term; your doctor may need to adjust your dose over time depending on your body weight and response to treatment. In general, the dose is calculated according to the following recommendations and is administered once a day:
Children and adolescents with:
- Growth hormone deficiency:
0.025 - 0.035 mg/kg body weight and day,
- Turner Syndrome:
0.045 - 0.050 mg/kg body weight and day,
- Chronic kidney disease:
0.045 - 0.050 mg/kg body weight and day,
- Small for gestational age at birth:
0.035 mg/kg body weight and day. Treatment should be discontinued after the first year of treatment if the growth rate is insufficient,
- SHOX gene deficiency:
0.045 - 0.050 mg/kg body weight and day.
Growth hormone deficiency in adults:
Treatment should be started with a low dose of 0.15-0.30 mg per day. It may be necessary to start with lower doses in patients who are overweight or elderly. The initial dose can be gradually increased according to individual needs. The total daily dose normally does not exceed 1 mg.
Dose requirements may decrease with age. Women, especially those on oral estrogen replacement therapy, may require higher doses than men.
If you use more Humatrope than you should
If you have injected more Humatrope than you should, consult your doctor.
- If you have injected too much Humatrope, your blood sugar may initially decrease and become too low (hypoglycemia) and then increase and become too high (hyperglycemia).
- If you inject too much Humatrope over a long period (years), you may experience overgrowth of some parts of your body such as ears, nose, jaw, hands, and feet (acromegaly).
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Humatrope
Do not inject a double dose to make up for forgotten doses. Continue with the prescribed dose. If you have forgotten to inject Humatrope and are unsure what to do, consult your doctor.
If you stop treatment with Humatrope
Ask your doctor before stopping treatment. Stopping or interrupting treatment with Humatrope prematurely may affect the success of treatment with Humatrope.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You may have any of the following side effects after injecting Humatrope:
The following criteria have been used for the classification of side effects:
Very common: may affect more than 1 in 10 people
Common: may affect up to 1 in 10 people
Uncommon: may affect up to 1 in 100 people
Rare: may affect up to 1 in 1,000 people
Very rare: may affect up to 1 in 10,000 people
Other possible side effects (frequency cannot be estimated from the available data)
Children | ||||
Common | Uncommon | Rare | Very rare | Other |
Pain at the injection site Swelling (edema) Increased blood sugar (hyperglycemia) Hypersensitivity to metacresol and/or glycerol Low thyroid hormone levels Development of antibodies against growth hormone Progression of scoliosis (an increase in the lateral curvature of the spine) | Weakness Type 2 diabetes mellitus Increased breast size (gynecomastia) | Severe or frequent headaches with nausea and/or vision problems that are signs of increased pressure in the brain (benign intracranial hypertension). Inform your doctor immediately if this occurs. Numbness and tingling (paresthesia) Localized muscle pain (myalgia) | Difficulty sleeping (insomnia) High blood pressure (hypertension) Sugar in the urine (glucosuria) | Hypersensitivity to the active substance |
Adults | ||||
Very common | Common | Uncommon | Rare | Other |
Headache Pain in the joints (arthralgia) | Pain at the injection site Swelling (edema) High blood sugar (hyperglycemia) Hypersensitivity to metacresol and/or glycerol Low thyroid hormone levels Difficulty sleeping (insomnia) Numbness and tingling (paresthesia) Numbness and tingling in the fingers and palm of the hand due to compression of the wrist nerve (carpal tunnel syndrome) Localized muscle pain (myalgia) High blood pressure (hypertension) Difficulty breathing (dyspnea) Temporary interruption of breathing during sleep (sleep apnea) | Weakness Increased breast size (gynecomastia) | Severe or frequent headaches with nausea and/or vision problems that are signs of increased pressure in the brain (benign intracranial hypertension). Inform your doctor immediately if this occurs. Sugar in the urine (glucosuria) | Type 2 diabetes mellitus Hypersensitivity to the active substance |
The effect of insulin may be reduced.
Leukemia has been reported in a small number of children who have been treated with growth hormone. However, there is no evidence that the incidence of leukemia is increased in patients receiving growth hormone.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Agency: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Humatrope
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton. The expiry date (EXP) is the last day of the month shown.
Do not use this medicine if you notice that the solution is not clear or if it contains particles.
Humatrope should always be stored in a refrigerator (between 2°C and 8°C). Do not freeze.
Once reconstituted, do not leave Humatrope out of the refrigerator for more than 30 minutes per day.
Once reconstituted, Humatrope can be used for up to a maximum of 28 days if stored in a refrigerator and not left out of the refrigerator for more than 30 minutes per day at room temperature.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Humatrope
Cartridge Powder
The active substance is somatropin. Each cartridge contains 6 mg, 12 mg, or 24 mg, depending on the concentration. Once reconstituted:
- Humatrope 6 mgcorresponds to 2.08 mg of somatropin per ml of solution
- Humatrope 12 mgcorresponds to 4.17 mg of somatropin per ml of solution
- Humatrope 24 mgcorresponds to 8.33 mg of somatropin per ml of solution
The other ingredients are: mannitol, glycine, disodium phosphate.
[During the manufacturing process, phosphoric acid or sodium hydroxide (or both) may have been used to adjust the acidity].
Sterile Diluent in the Syringe
The pre-filled syringe diluent contains: glycerol, metacresol, water for injectable preparations. [During the manufacturing process, hydrochloric acid or sodium hydroxide (or both) may have been used to adjust the acidity].
Appearance of the Product and Package Contents
Humatrope 6 mg: |
Package size of 1, 5, and 10 |
Humatrope 12 mg: |
Package size of 1, 5, and 10 |
Humatrope 24 mg: |
Package size of 1, 5, and 10 |
Not all pack sizes may be marketed
Marketing Authorization Holder
LILLY, S.A. Avda. de la Industria, 30. 28108 Alcobendas (Madrid).
Manufacturer
Lilly France, S.A.S. Rue du Colonel Lilly, 67640 Fegersheim (France).
For any information about this medicinal product, please contact the Marketing Authorization Holder (or their local representative):
Lilly, S.A. Avda. de la Industria, 30. 28108 Alcobendas (Madrid).
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
In the Member States of the European Economic Area where this product is authorized, it is authorized under the name of “Humatrope”, except in France where it is authorized as “Umatrope”.
How to Inject Humatrope 6 mg/12 mg/24 mg
The following instructions explain how to inject Humatrope. Read the instructions carefully and follow them step by step.
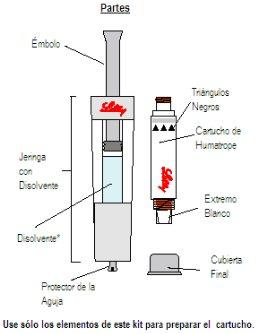
Getting Started: You will need five pieces:
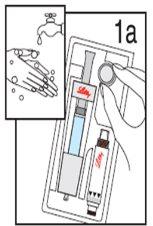
Wash your hands before proceeding with the next steps. |
|
*Note: The liquid is colorless. It is shown in blue only for illustration purposes. |
The following steps will guide you in preparing your new cartridge for use | ||
Step 1 Unpacking
| ||
Remove ALLcontents from the box. Note: This product is designed to be used by both left- and right-handed people. Please use the hand that is most comfortable for you. | Hold the needle protector, located at the end of the diluent syringe. | Remove the needle protector and discard it. DO NOTpress the plunger yet. Sometimes a drop of liquid may come out. It is not necessary to release the air from the diluent syringe. |
Steps 2 and 3 Cartridge Placement
| ||
Hold the cartridge with the black triangles facing up. Place the cartridge and the diluent syringe in a straight line. DO NOTinsert the cartridge at an angle. | PUSHthe cartridge STRAIGHTuntil it clicks ANDuntil the black triangles are CLOSED. You may hear or feel a “click”. DO NOTturn the cartridge. | |
| ||
Hold the diluent syringe and the cartridge together with BOTH HANDS. Press and release the plunger 2 or 3 times until the diluent is in the cartridge. | Remove your thumb from the plunger and check that the diluent syringe is empty (it is normal for small drops of diluent to remain in the syringe). | |
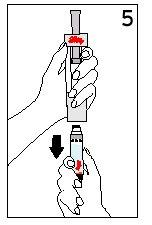
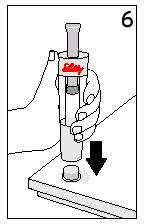
Steps 5 and 6 Cartridge Removal and Diluent Disposal
| ||
With your thumb AWAYfrom the plunger, remove the cartridge from the diluent syringe | Place the final cover on a hard, flat surface. Push the diluent syringe onto the final cover and immediately dispose of the diluent syringe following your doctor's instructions. | |
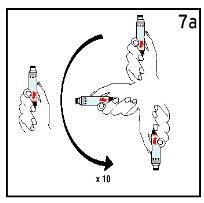
Step 7 Gentle Mixing
| ||
Mix the cartridge gently 10 times and let it rest for 3 minutes. DO NOT SHAKE. | Observe the solution. The Humatrope solution should be clear. | |
Step 8 Injection of Humatrope using a suitable pen injection system.
|
Humatrope is a registered trademark of Eli Lilly and Company Limited
Date of the last revision of this leaflet: July 2021
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HUMATROPE 24 mg POWDER AND SOLVENT FOR INJECTIONDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 5.3 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 0.2 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Online doctors for HUMATROPE 24 mg POWDER AND SOLVENT FOR INJECTION
Discuss questions about HUMATROPE 24 mg POWDER AND SOLVENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





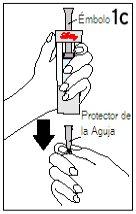
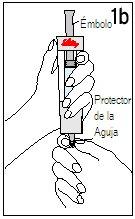
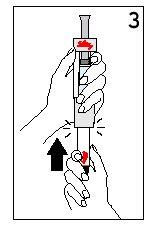
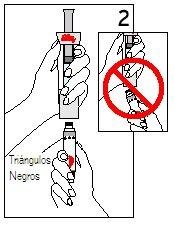
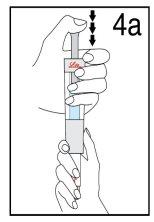
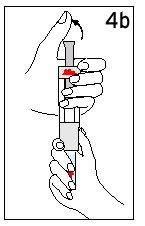 Step 4 Mixing Humatrope
Step 4 Mixing Humatrope