
GENOTONORM KABIPEN 5,3 mg POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar GENOTONORM KABIPEN 5,3 mg POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
GENOTONORM KABIPEN 5,3 mg y 12 mg polvo y disolvente para solución inyectable
somatropina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Contenido del prospecto:
- Qué es Genotonorm Kabipen y para qué se utiliza
- Qué necesita saber antes de empezar a usar Genotonorm Kabipen
- Cómo usar Genotonorm Kabipen
- Posibles efectos adversos
- Conservación de Genotonorm Kabipen
Contenido del envase e información adicional
1. Qué es Genotonorm Kabipen y para qué se utiliza
Genotonorm Kabipen es una hormona recombinante de crecimiento humano (también conocida como somatropina). Tiene la misma estructura que la hormona de crecimiento humana, la cual es necesaria para el crecimiento de los huesos y de los músculos. También ayuda a que el tejido graso y muscular se desarrollen en las cantidades adecuadas. El que sea recombinante significa que no procede ni de tejido humano ni animal.
En niños, Genotonorm Kabipen se utiliza para el tratamiento de trastornos del crecimiento:
- Si no crece de forma adecuada o si no posee suficiente hormona de crecimiento propia.
- En caso de síndrome de Turner. El síndrome de Turner es una alteración cromosómica que se da en las niñas y puede afectar al crecimiento – su médico le indicará si lo padece.
- En caso de insuficiencia de los riñones crónica. Si el riñón pierde su capacidad para funcionar adecuadamente, el crecimiento puede verse afectado.
- En caso de síndrome de Prader-Willi (una alteración cromosómica). La hormona de crecimiento le ayudará a crecer si aún se encuentra en periodo de crecimiento y también mejorará la composición corporal. El exceso de grasa disminuirá y la pérdida de masa muscular mejorará.
- En caso de haber nacido pequeño o con poco peso. La hormona de crecimiento puede ayudarle a crecer si aún no ha sido capaz de alcanzar o mantener el crecimiento normal a la edad de 4 años o posterior.
En adultos, Genotonorm Kabipen se utiliza para el tratamiento de personas con una marcada deficiencia de hormona de crecimiento. Esto puede iniciarse en la edad adulta, o puede haberse iniciado en la infancia y continuar hasta la edad adulta.
Si usted ha estado en tratamiento con Genotonorm Kabipen por una deficiencia de hormona de crecimiento durante la infancia, se debe reevaluar la hormona de crecimiento después de completar la fase de crecimiento. Si se confirma la deficiencia severa de hormona de crecimiento, su médico le propondrá la continuación del tratamiento con Genotonorm Kabipen.
Este medicamento sólo se lo puede prescribir un médico con experiencia en el tratamiento con hormona de crecimiento y que le haya confirmado su diagnóstico.
2. Qué necesita saber antes de empezar a usar Genotonorm Kabipen
No use Genotonorm Kabipen y contacte con su médico si
- Es alérgico (hipersensible) a la somatropina o a alguno de los demás componentes de Genotonorm Kabipen.
- Padece un tumor activo (cáncer). Los tumores deben estar inactivos y debe haber finalizado el tratamiento antitumoral antes de empezar el tratamiento con Genotonorm.
- Está gravemente enfermo (por ejemplo, complicaciones tras una cirugía a corazón abierto, cirugía abdominal, insuficiencia respiratoria aguda, traumatismo accidental o alguna situación similar). Si está a punto de someterse o se ha sometido a una operación importante o va a ingresar en el hospital por cualquier razón, dígaselo a su médico y recuerde al resto de médicos que le examinen que utiliza hormona de crecimiento.
- Ya ha finalizado su periodo de crecimiento (epífisis cerradas) y se le había prescrito Genotonorm Kabipen para estimular el crecimiento.
Tenga especial cuidado con Genotonorm Kabipen y contacte con su médico
- Si tiene riesgo de desarrollar diabetes, su médico vigilará los niveles de azúcar en sangre durante el tratamiento con Genotonorm Kabipen.
- Si usted tiene diabetes, ha de vigilar cuidadosamente sus niveles de azúcar en sangre durante el tratamiento con Genotonorm Kabipen y ver los resultados con su médico para determinar si necesita cambiar la dosis de sus medicamentos para la diabetes.
- Tras el inicio del tratamiento con Genotonorm Kabipen, algunos pacientes pueden necesitar comenzar tratamiento con hormona tiroidea.
- Si usted está recibiendo tratamiento con hormonas tiroideas, puede ser necesario ajustar la dosis de la hormona tiroidea.
- Si usted está en tratamiento con hormona de crecimiento para estimular el crecimiento y cojea, o si empieza a cojear porque tiene dolor en la cadera durante el tratamiento con hormona de crecimiento, debe informar a su médico.
- Si se produce un aumento de la presión intracraneal (con síntomas tales como fuertes dolores de cabeza, problemas de visión o vómitos) debe informar a su médico sobre ello.
- Si su médico le confirma que ha desarrollado inflamación de los músculos cerca del área de inyección a causa del conservante metacresol, debe utilizar otra presentación de Genotonorm que no contenga metacresol.
- Si usted está recibiendo Genotonorm Kabipen por una deficiencia de hormona de crecimiento tras un tumor previo (cáncer), se le debe someter a una revisión periódica por posibles recurrencias del tumor o cualquier otro cáncer.
- Si sufre dolor abdominal que se vuelve más intenso, debe informar a su médico.
- La experiencia en pacientes de más de 80 años de edad es limitada. Las personas de edad avanzada pueden ser más sensibles a la acción de Genotonorm Kabipen, y por tanto, pueden tener más predisposición a desarrollar efectos adversos.
Niños con insuficiencia renal crónica:
- Su médico examinará su función renal y su velocidad de crecimiento antes de iniciar el tratamiento con Genotonorm Kabipen. El tratamiento médico para su enfermedad renal debe continuar. El tratamiento con Genotonorm Kabipen debería suspenderse en caso de trasplante de riñón.
Niños con síndrome de Prader-Willi:
- Su médico le dará unas restricciones en la dieta para que controle su peso.
- Su médico le realizará una exploración antes de comenzar el tratamiento con Genotonorm Kabipen para determinar si padece obstrucción de las vías respiratorias altas, apnea del sueño (cuando la respiración se interrumpe durante el sueño) o infecciones respiratorias.
- Si durante el tratamiento presenta signos de obstrucción de las vías respiratorias altas (incluyendo el comienzo o aumento de ronquidos), su médico necesitará examinarle y podría interrumpir el tratamiento con Genotonorm Kabipen.
- Durante el tratamiento su médico vigilará cualquier signo de escoliosis, un tipo de deformación de la columna.
- Si durante el tratamiento desarrolla una infección pulmonar, dígaselo a su médico para que le pueda tratar la infección.
Niños nacidos pequeños o con peso bajo:
- Si usted nació pequeño o con peso bajo y tiene una edad entre 9 y 12 años, consulte específicamente a su médico sobre la pubertad y el tratamiento con este producto.
- Su médico realizará análisis de azúcar y de insulina en sangre antes de comenzar el tratamiento y una vez al año mientras éste dure.
- El tratamiento ha de continuar hasta que finalice su fase de crecimiento.
Uso en deportistas
Este medicamento contiene somatropina que puede producir un resultado positivo en las pruebas de control de dopaje.
Uso de otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente cualquier otro medicamento, incluso los adquiridos sin receta.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Genotonorm.
Si está recibiendo tratamiento de sustitución con glucocorticoides, debe consultar con su médico regularmente ya que puede ser necesario un ajuste de su dosis de glucocorticoide.
Informe a su médico si está utilizando:
- medicamentos para el tratamiento de la diabetes
- hormonas tiroideas
- hormonas sintéticas adrenales (corticosteroides)
- estrógenos administrados por vía oral u otras hormonas sexuales
- ciclosporina (medicamento que debilita el sistema inmunológico tras un trasplante)
- medicamentos para el control de la epilepsia (anticonvulsivantes)
Su médico puede necesitar realizar un ajuste de la dosis de estos medicamentos o de la dosis de Genotonorm Kabipen.
Embarazo y lactancia
No utilice Genotonorm Kabipen si está embarazada, cree que podría estar embarazada o está intentando quedarse embarazada.
Consulte con su médico antes de utilizar este medicamento mientras está en periodo de lactancia.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
Genotonorm Kabipen contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Genotonorm Kabipen
Dosis recomendada
La dosis depende de su superficie corporal, de la patología para la cual está siendo tratado y de cómo funciona su hormona de crecimiento. Cada persona es diferente. Su médico le indicará su dosis individualizada de Genotonorm Kabipen en miligramos (mg) bien en función de su peso corporal en kilogramos (kg) o de su superficie corporal calculada a partir de su altura y su peso en metros cuadrados (m2), así como el esquema de su tratamiento. No cambie ni la dosis ni el esquema de tratamiento sin consultar con su médico.
Niños con deficiencia de hormona de crecimiento:
0,025-0,035 mg/kg de peso corporal al día o 0,7-1,0 mg/m2 de superficie corporal al día. Se pueden utilizar dosis más altas. Cuando la deficiencia de hormona de crecimiento continúa en la adolescencia, se debe continuar el tratamiento con Genotonorm Kabipen hasta que se complete el desarrollo físico.
Niños con síndrome de Turner:
0,045-0,050 mg/kg de peso corporal al día o 1,4 mg/m2 de superficie corporal al día.
Niños con insuficiencia renal crónica:
0,045-0,050 mg/kg de peso corporal al día o 1,4 mg/m2 de superficie corporal al día. Si la velocidad de crecimiento es demasiado baja puede ser necesario utilizar dosis más altas. Puede ser necesario un ajuste de dosis tras 6 meses de tratamiento.
Niños con síndrome de Prader-Willi:
0,035 mg/kg de peso corporal al día o 1,0 mg/m2 de superficie corporal al día. La dosis diaria no debe exceder de 2,7 mg. Este tratamiento no debe utilizarse en aquellos niños cuya fase de crecimiento haya prácticamente finalizado tras la pubertad.
Niños nacidos pequeños o con menor peso de lo esperado y con trastornos del crecimiento:
0,035 mg/kg de peso corporal al día o 1,0 mg/m2 de superficie corporal al día. Es importante continuar el tratamiento hasta alcanzar la talla final. El tratamiento debe suspenderse tras el primer año si no hay respuesta o si se alcanza la talla final y el crecimiento ha finalizado.
Adultos con deficiencia de hormona de crecimiento:
Si continúa utilizando Genotornorm tras el tratamiento durante la infancia, debe empezar con una dosis de 0,2-0,5 mg al día. Esta dosis debe ser incrementada o disminuida de forma gradual de acuerdo a los resultados analíticos, así como a la respuesta clínica y los efectos adversos.
Si la deficiencia de hormona de crecimiento se inicia durante la edad adulta, debe iniciarse con 0,15-0,3 mg al día. Esta dosificación debe incrementarse de forma gradual en función de los resultados analíticos así como de la respuesta clínica y los efectos adversos. La dosis diaria de mantenimiento rara vez excede de 1,0 mg al día. Las mujeres pueden requerir dosis más altas que los hombres. La dosificación ha de ser monitorizada cada 6 meses. Los pacientes mayores de 60 años deben empezar con dosis de 0,1-0,2 mg al día e ir incrementándola lentamente según las necesidades individuales. Debe utilizarse la dosis mínima efectiva. La dosis de mantenimiento rara vez excede de 0,5 mg al día. Siga las instrucciones indicadas por su médico.
Inyección de Genotonorm Kabipen
Este medicamento se utiliza por vía subcutánea. Esto significa que se inyecta por medio de una pequeña aguja en el tejido graso, justo por debajo de la piel. Su médico le enseñará como utilizarlo. Siempre utilice el medicamento tal y como su médico le ha indicado. Consulte con su médico o farmacéutico si tiene alguna duda.
Las instrucciones de uso del dispositivo precargado GoQuick también se suministran en la caja del dispositivo precargado.
Las instrucciones para utilizar Genotonorm Kabipen en vial de doble cámara junto con Genotonorm Pen se suministran en el estuche del dispositivo de inyección.
Consulte estas instrucciones antes de utilizar su medicamento.
Cuando utilice un dispositivo precargado o un dispositivo de inyección Genotonorm Pen, deberá colocar la aguja en el dispositivo antes de realizar la mezcla. Utilice una aguja nueva con cada inyección. Las agujas no se deben reutilizar.
- Preparación de la inyección:
Puede sacar el medicamento de la nevera media hora antes de la inyección. Esto permite que se atempere un poco y que la inyección le sea más cómoda.
El dispositivo precargado GoQuick contiene un vial de doble cámara que tiene la hormona de crecimiento y el disolvente líquido. La hormona de crecimiento y el disolvente líquido se mezclan al girar el vial (ver los pasos en detalle en las Instrucciones de Uso). No se necesita ningún dispositivo adicional.
Genotonorm también se presenta en vial de doble cámara que contiene la hormona de crecimiento y el disolvente líquido para utilizar con el dispositivo Genotonorm Pen. La hormona de crecimiento y el disolvente líquido en el vial de doble cámara, se pueden mezclar enroscando juntos el dispositivo Genotonorm Pen.
En ambos casos, tanto para el dispositivo precargado GoQuick como para el vial de doble cámara, disolver el polvo inclinándolo suavemente hasta que el polvo quede completamente disuelto.
Cuando esté mezclando Genotonorm Kabipen, NO AGITE la solución. Mézclela suavemente. Si agita la solución puede hacer que la hormona del crecimiento haga espuma y dañe el medicamento. Observe la solución y no se la inyecte si observa turbidez o que hay partículas en ella.
- Inyección de Genotonorm Kabipen:
Recuerde lavarse las manos primero y limpiarse la piel en el lugar de inyección.
Póngase la inyección de hormona de crecimiento a la misma hora todos los días. Un buen momento es la hora de irse a la cama ya que es fácil de recordar. También es normal tener un nivel más alto de hormona de crecimiento por la noche.
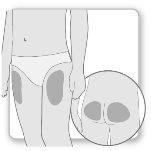
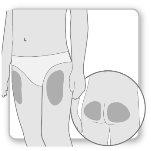
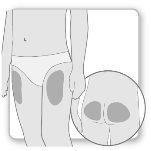
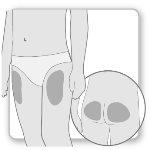
La mayor parte de los pacientes utilizan los muslos o los glúteos para la inyección. Póngase la inyección donde le haya enseñado su médico. El tejido graso de la piel puede disminuir de tamaño en la zona de inyección. Para evitarlo, cambie el punto de inyección cada vez. Así le dará tiempo a la piel y a la zona bajo la piel a recuperarse entre una inyección y otra antes de volver a inyectarse en el mismo punto.
Recuerde guardar el medicamento en la nevera inmediatamente después de la inyección.
Si usa más Genotonorm Kabipen del que debe
Si se inyecta más cantidad de la que debiera, consulte inmediatamente a su médico o farmacéutico. Los niveles de azúcar en sangre pueden descender bruscamente y posteriormente aumentar a niveles demasiado altos. Usted puede sentirse agitado, sudoroso, somnoliento o sentirse extraño y podría marearse.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 5620420, indicando el medicamento y la cantidad ingerida.
Si olvidó administrarse Genotonorm Kabipen
No se administre una dosis doble para compensar las dosis olvidadas.
Es mejor que se inyecte la hormona de crecimiento de forma regular. Si olvidó administrarse una dosis, póngase la siguiente inyección a la hora correspondiente del día siguiente. Anote las inyecciones olvidadas y dígaselo a su médico en la siguiente revisión.
Si interrumpe el tratamiento con Genotonorm Kabipen
Consulte con su médico antes de interrumpir el tratamiento con este medicamento.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos frecuentes y muy frecuentes en los adultos pueden comenzar en los primeros meses de tratamiento y desaparecer de manera espontánea o cuando se reduce la dosis.
Los efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 pacientes) incluyen:
En adultos:
- Dolor en las articulaciones.
- Retención de líquidos (que se manifiesta en forma de dedos hinchados o hinchazón de los tobillos).
Los efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 pacientes) incluyen:
En niños:
- Dolor en las articulaciones.
- Enrojecimiento, picor o dolor temporal en el lugar de inyección.
En adultos:
- Entumecimiento/hormigueo.
- Dolor o sensación de quemazón en las manos o en las axilas (conocido como síndrome del túnel carpiano).
- Rigidez en brazos y piernas, dolor muscular.
Los efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas) incluyen:
En niños:
- Leucemia (se ha notificado en un pequeño número de pacientes con deficiencia de hormona de crecimiento, algunos de los cuales han recibido tratamiento con somatropina. No obstante, no existe evidencia de que la incidencia de leucemia esté aumentada en los receptores de hormona de crecimiento sin factores predisponentes).
- Aumento de la presión intracraneal (que causa síntomas tales como fuerte dolor de cabeza, problemas de visión o vómitos).
- Entumecimiento/hormigueo.
- Erupción.
- Picor.
- Habones en la piel con picor.
- Dolor muscular.
- Aumento de tamaño del pecho (ginecomastia).
- Retención de líquidos (que se manifiesta en forma de dedos hinchados o hinchazón de los tobillos, durante un corto periodo de tiempo al comienzo del tratamiento).
En adultos:
- Aumento de tamaño del pecho (ginecomastia).
Frecuencia no conocida: no puede estimarse a partir de los datos disponibles:
- Diabetes tipo II.
- Hinchazón en la cara.
- Dolor de cabeza.
- Disminución de las concentraciones de la hormona cortisol en la sangre.
En niños:
- Rigidez en brazos y piernas.
En adultos:
- Aumento de la presión intracraneal (que causa síntomas tales como fuerte dolor de cabeza, problemas de visión o vómitos).
- Erupción.
- Picor.
- Habones en la piel con picor.
- Enrojecimiento, picor o dolor en el lugar de inyección.
Formación de anticuerpos frente a la hormona de crecimiento inyectada, aunque esto no parece que afecte a la acción de la hormona de crecimiento.
La piel que hay alrededor del área de inyección puede volverse rugosa e irregular, pero esto no debería ocurrir si la inyección se realiza en un lugar diferente cada vez.
El conservante metacresol puede producir como efecto adverso muy raro la inflamación de los músculos próximos al lugar de inyección. Si su médico le confirma que ha desarrollado este efecto adverso debería usar Genotonorm sin metacresol.
Ha habido casos raros de muerte súbita en pacientes con síndrome de Prader-Willi. No obstante, no se ha podido establecer relación entre estos casos y el tratamiento con este medicamento .
Si presenta molestia o dolor de cadera o rodilla mientras recibe tratamiento con Genotonorm, su médico puede plantearse la posibilidad de que sufra epifisiólisis femoral superior o enfermedad de Legg-Calvé-Perthes.
Otros posibles efectos secundarios relacionados con el tratamiento con hormona de crecimiento son los siguientes.
Usted (o su hijo) puede presentar aumento de los niveles de azúcar en sangre o disminución de las concentraciones de hormona tiroidea. Su médico puede realizar pruebas para determinarlo y, en caso necesario, le recetará el tratamiento adecuado. Ocasionalmente, se ha notificado inflamación del páncreas en pacientes tratados con hormona de crecimiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaRAM.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Genotonorm Kabipen
Mantener fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase como MM/AAAA. La fecha de caducidad es el último día del mes que se indica.
Antes de la reconstitución
Conservar en nevera (2ºC- 8ºC). Conservar el vial de doble cámara en el embalaje exterior para protegerlo de la luz.
Antes de abrirlo, el medicamento puede mantenerse fuera de la nevera durante un periodo máximo de 1 mes a temperatura no superior a 25ºC, pero transcurrido este plazo debe desecharse.
Después de la reconstitución
Conservar en nevera (2ºC- 8ºC) durante un periodo máximo de 28 días. No congelar. Conservar el dispositivo precargado GoQuick en el embalaje exterior, o el vial de doble cámara en el estuche del dispositivo de inyección, para protegerlo de la luz.
No utilice este medicamento si observa partículas o si la solución está turbia.
No congele ni exponga este medicamentoal hielo. Si se congela, no la utilice.
No tire nunca ni las agujas ni los viales vacíos a la basura normal. Cuando haya terminado de utilizar la aguja, debe deshacerse de ella con cuidado en un recipiente especial para agujas, de forma que nadie pueda utilizarla ni pincharse.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda, pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Genotonorm Kabipen
- El principio activo es somatropina*.
- Un vial contiene, 5,3 mg o 12 mg de somatropina*.
- Después de la reconstitución, la concentración de somatropina* es 5,3 mg o 12 mg por ml.
- Los demás componentes del polvo son: glicina (E640), manitol (E421), hidrógeno fosfato de sodio anhidro (E339) y dihidrógeno fosfato de disodio anhidro (E339) (ver sección 2 “Genotonorm Kabipen contiene sodio”).
- Los ingredientes del disolvente son: agua para preparaciones inyectables, manitol (E421) y metacresol.
*Obtenida en células de Escherichia colipor tecnología de ADN recombinante.
Aspecto de Genotonorm Kabipen y contenido del envase
Polvo y disolvente para solución para inyección, en un vial de doble cámara que contiene el polvo en una sección y el disolvente en la otra (5,3 mg/ml o 12 mg/ml). El vial puede venir incluido en un dispositivo precargado. Tamaños de envase: 1 o 5 dispositivo(s) precargado(s), o bien 1, 5 o 20 vial(es).
Puede que solamente estén comercializados algunos tamaños de envases.
El polvo es blanco y el disolvente transparente.
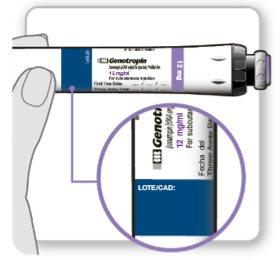
Los viales deben utilizarse con un dispositivo de inyección Genotonorm Kabipen específico. Los viales de Genotonorm Kabipen tienen un código de color, y deben utilizarse con el dispositivo de inyección Genotonorm Kabipen que coincida con el código de color, para proporcionar la dosis correcta: el vial de Genotonorm Kabipen 5,3 mg (azul) se debe utilizar con el dispositivo de inyección Genotonorm Kabipen 5,3 (azul). El vial de Genotonorm Kabipen 12 mg (violeta) se debe utilizar con el dispositivo de inyección Genotonorm Kabipen 12 (violeta).
Las instrucciones de uso del dispositivo se incluyen en su embalaje. Si no tiene un dispositivo de inyección puede pedírselo a su médico.
Titular de la autorización de comercialización
Pfizer, S.L.
Avda. de Europa 20 B
Parque Empresarial La Moraleja
28108 Alcobendas (Madrid), España.
Responsable de la fabricación
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Bélgica
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo y en el Reino Unido (Irlanda del Norte) con los siguientes nombres:
Genotropin: Austria, Dinamarca, Finlandia, Alemania, Grecia, Irlanda, Italia, Holanda, Portugal, Suecia, Reino Unido (Irlanda del Norte).
Genotonorm: Bélgica, Francia, Luxemburgo.
Genotonorm Kabipen: España.
Fecha de la última revisión de este prospecto:Mayo 2024
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
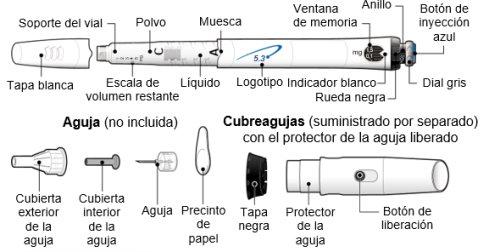
INSTRUCCIONES DE USO DE GENOTONORM KABIPEN GOQUICK® Lea estas instrucciones al completo antes de utilizar el dispositivo GoQuick. Si tiene alguna duda acerca de la dosis o del tratamiento con Genotonorm, llame a su médico o enfermero. Acerca de GoQuick GoQuick es un dispositivo de inyección precargado, multidosis y desechable que contiene 5,3 mg de somatropina. El dispositivo puede administrar dosis de 0,1 mg a 1,5 mg del medicamento. Cada clic de la rueda negra cambia la dosis en 0,05 mg. El vial de Genotonorm incorporado en el dispositivo se mezcla una única vez, cuando empiece a usar un nuevo dispositivo. Nunca debe cambiar el vial. Cuando el dispositivo esté vacío, ha de empezar a utilizar uno nuevo. El dispositivo tiene memoria de dosis. La dosis se ajusta cada vez que se vaya a utilizar un nuevo dispositivo. Después, el dispositivo le permite preparar la misma dosis establecida en cada inyección. Esto evitará que extraiga una dosis mayor de la establecida. Información importante
Conservación y eliminación
Siga las normativas de salud y seguridad locales para desechar (tirar) el dispositivo. Pregunte a su médico o enfermero si no está seguro de qué hacer. Partes del dispositivo GoQuick
Las agujas para el dispositivo no están incluidascon el dispositivo GoQuick. Tendrá que adquirir agujas para dispositivos de hasta 8 mm de longitud en la farmacia.
Preparación y uso de un nuevo dispositivo GoQuick |
(-:Step 1.)=100%(Paso 1.:-)(-:Attach the Needle)=89%(Preparación
(-:Push the needle onto the cartridge holder tip.)=81%((-:Do not overtighten.)=100%( (-:Leave both needle covers on the needle.)=0%(<0} |
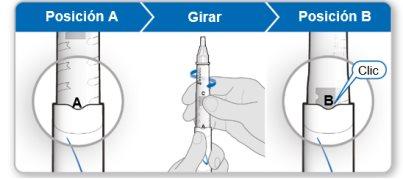
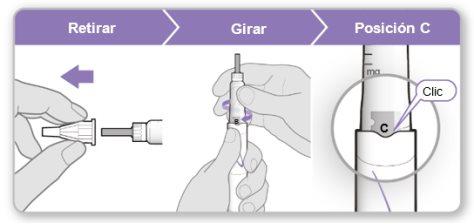
{0>Step 2.<}100{>Paso 2.<0}{0>Mix the Genotropin<}0{>Elegir el lugar de inyección<0}
<0} |
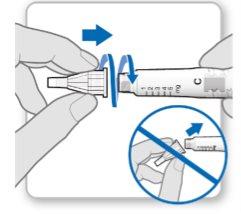
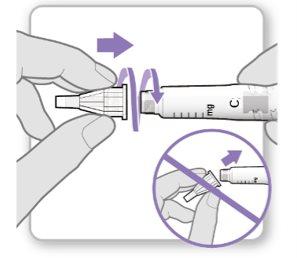
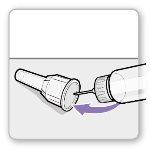
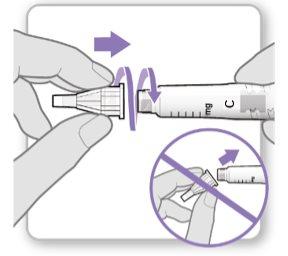
Paso 3.Fijar una aguja nueva<0}
Nota:Tenga cuidado de no fijar la aguja en ángulo. Esto puede causar escapes en el dispositivo.
<0} |
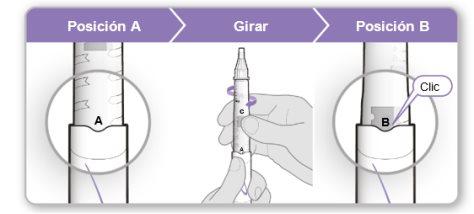
Paso 4.Mezclar el contenido del vial de Genotonorm<0}
|
|
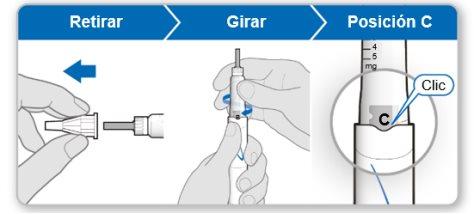
Paso5Extraer elaire del dispositivo
Nota:Debería ver una cubierta interior de la aguja después de haber quitado la cubierta exterior. Si no ve esto, intente fijar la aguja otra vez.
|
Paso 6.Purgar el dispositivo
La purga elimina el aire restante empujando una pequeña cantidad de líquido fuera del dispositivo. La dosis inicial es de 0,1 mg y es diferente de la dosis que le ha recetado su médico o enfermero. Purgue el dispositivo únicamente la primera vez que lo utilice.
Precaución: Notoque la aguja para evitar pincharse.
|
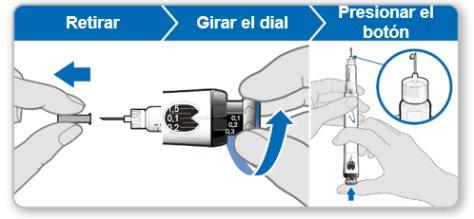
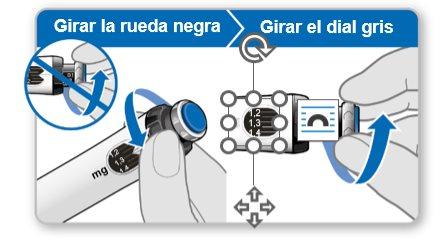
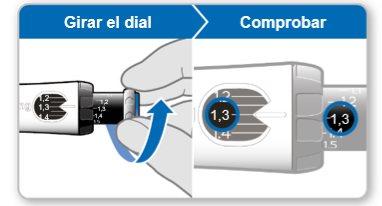
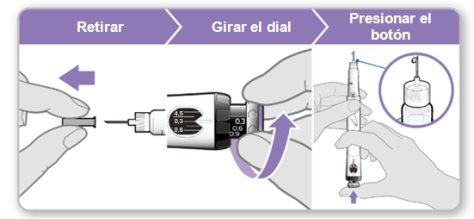
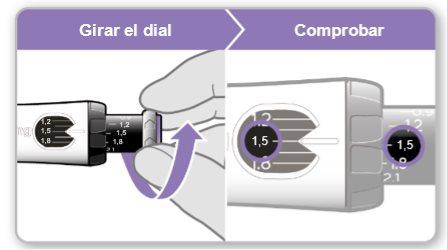
Paso 7.Ajustar y preparar su dosis
La primera vez que utilice el dispositivo ajustará la dosis que le haya recetado su médico o enfermero. No es necesario que vuelva a ajustar la dosis hasta que comience a usar un nuevo dispositivo o hasta que su médico o enfermero se lo indique.
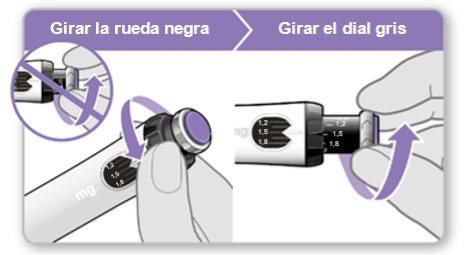
Nota:Si no puede girar la rueda negra, presione el botón de inyección azul hasta que deje de hacer clic. Después intente ajustar su dosis otra vez. Tenga en cuenta que saldrá líquido de la aguja.
|
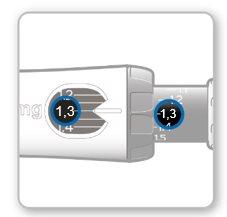
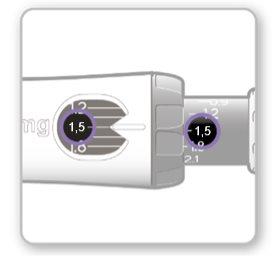
Paso8Comprobar su dosis
Su dosis debe quedar alineadacon el indicador blanco.
|
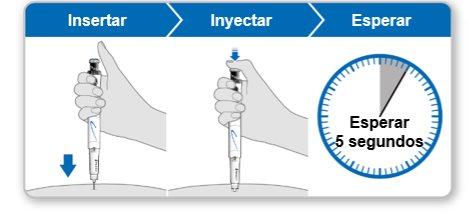
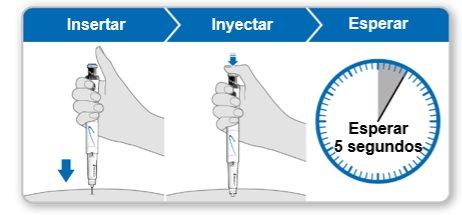
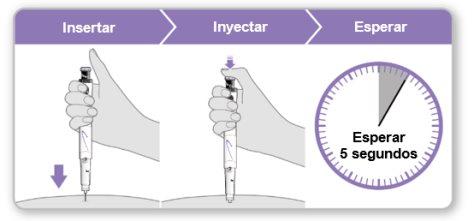
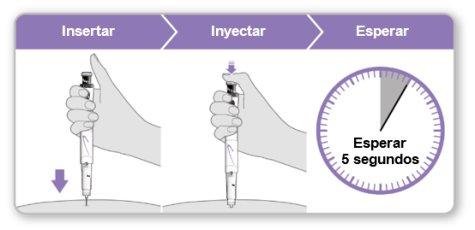
Paso9Administrar la inyección de Genotonorm Kabipen
Nota:Si ve una gota de líquido en el lugar de inyección o en la punta de la aguja, con la siguiente inyección intente presionar el botón de inyección azul durante más tiempo antes de sacar la aguja de la piel. |
Paso10Retirar la aguja
Conservar el dispositivo en la nevera hasta la siguiente inyección. |
Uso habitual (diario) del dispositivo GoQuick | |||
Paso1Preparación
| |||
Paso2Elegir el lugar de inyección
| |||
Paso3Fijar un aguja nueva
| |||
Paso4Preparar su dosis
Nota:Si la dosis a extraer es inferior, el dispositivo no dispone de una dosis completa de Genotonorm. Siga las instrucciones que su médico o enfermero le haya dado en caso de que no quede una dosis completa en el dispositivo. O comuníquese con su médico o enfermero para obtener asesoramiento. | |||
Paso5Administrar la inyección de Genotonorm Kabipen
Nota:Si ve una gota de líquido en el lugar de inyección o en la punta de la aguja, con la siguiente inyección intente presionar el botón de inyección azul durante más tiempo antes de sacar la aguja de la piel. | |||
| |||
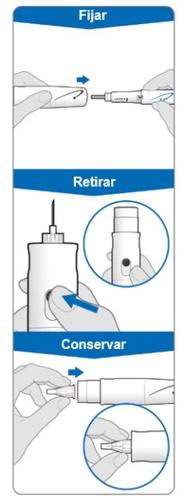
El cubreagujas es un artículo opcional que se suministra por separado para ocultar la aguja durante la inyección. Fijar el cubreagujas: Fijar el cubreagujas después del paso 5 (Ajuste y uso de un nuevo dispositivo GoQuick) para evitar pincharse.
Para retirar la aguja con el cubreagujas colocado:
Para retirar el cubreagujas:
|
INSTRUCCIONES DE USO DE GENOTONORM KABIPEN GOQUICK® |
Lea estas instrucciones al completo antes de utilizar el dispositivo GoQuick. Si tiene alguna duda acerca de la dosis o del tratamiento con Genotonorm Kabipen, llame a su médico o enfermero. Acerca de GoQuick GoQuick es un dispositivo de inyección precargado, multidosis y desechable que contiene 12 mg de somatropina. El dispositivo puede administrar dosis de 0,3 mg a 4,5 mg de Genotonorm Kabipen. Cada clic de la rueda negra cambia la dosis en 0,15 mg. El vial de Genotonorm incorporado en el dispositivo se mezcla una única vez, cuando empiece a usar un dispositivo nuevo. Nunca debe cambiar el vial. Cuando el dispositivo esté vacío, ha de empezar a utilizar uno nuevo. El dispositivo tiene memoria de dosis. La dosis se ajusta cada vez que se vaya a utilizar un nuevo dispositivo. Después, el dispositivo le permite preparar la misma dosis establecida en cada inyección. Esto evitará que usted extraiga una dosis mayor de la establecida. Información importante
Conservación y eliminación
|
Partes del dispositivo GoQuick
Las agujas para dispositivos no están incluidascon el dispositivo GoQuick. Tendrá que adquirir agujas para dispositivos de hasta 8 mm de longitud en la farmacia.
|
Preparación y uso de un nuevo dispositivo GoQuick |
Paso 1Preparación
Paso2Elegir el lugar de inyección
Paso3Fijar una aguja nueva
Nota:Tenga cuidado de no fijar la aguja en ángulo. Esto puede causar escapes en el dispositivo.
|
Paso4.Mezclar el contenido del vial de Genotonorm
| |
Paso5Extraer elaire del dispositivo
Nota:Debería ver una cubierta interior de la aguja después de haber quitado la cubierta exterior. Si no ve esto, intente fijar la aguja otra vez.
| |
Paso6Purgar el dispositivo
La purga elimina el aire restante empujando una pequeña cantidad de líquido fuera del dispositivo. La dosis inicial es de 0,3 mg y es diferente de la dosis que le ha recetado su médico o enfermero. Purgue el dispositivo únicamente la primera vez que lo utilice.
Precaución: Notoque la aguja para evitar pincharse.
| |
Paso7Ajustar y preparar su dosis
La primera vez que utilice el dispositivo ajustará la dosis que le haya recetado su médico o enfermero. No es necesario que vuelva a ajustar la dosis hasta que comience a usar un nuevo dispositivo o hasta que su médico o enfermero se lo indique.
Nota: Si no puede girar la rueda negra, presione el botón de inyección violeta hasta que deje de hacer clic. Después intente ajustar su dosis otra vez. Tenga en cuenta que saldrá líquido de la aguja.
| |
Paso8Comprobar su dosis
Su dosis debe quedar alineadacon el indicador blanco.
| |
Paso9Administrar la inyección de Genotonorm Kabipen
Nota:Si ve una gota de líquido en el lugar de inyección o en la punta de la aguja, con la siguiente inyección intente presionar el botón de inyección violeta durante más tiempo antes de sacar la aguja de la piel. | |
Paso10Retirar la aguja
| |
Uso habitual (diario) del dispositivo GoQuick | |
Paso1Preparación
| |
Paso2Elegir el lugar de inyección
Paso3Fijar una aguja nueva
Nota:Tenga cuidado de no fijar la aguja en ángulo. Esto puede causar escapes en el dispositivo.
| |
Paso4Preparar su dosis
| |
Nota:Si la dosis a extraer es inferior, el dispositivo no dispone de una dosis completa de Genotonorm. Siga las instrucciones que su médico o enfermero le haya dado en caso de que no quede una dosis completa en el dispositivo. O comuníquese con su médico o enfermero para obtener asesoramiento. | |
Paso5Administrar la inyección de Genotonorm Kabipen
Nota:Si ve una gota de líquido en el lugar de inyección o en la punta de la aguja, con la siguiente inyección intente presionar el botón de inyección violeta durante más tiempo antes de sacar la aguja de la piel. | |
Paso6Retirar la aguja
| |
Precaución: Notoque la aguja para evitar pincharse.
Uso del cubreagujas (opcional) El cubreagujas es un artículo opcional que se suministra por separado para ocultar la aguja durante la inyección.
Fijar el cubreagujas después del paso 5 (Ajuste y uso de un nuevo dispositivo GoQuick) para evitar pincharse.
Para retirar la aguja con el cubreagujas colocado:
Para retirar el cubreagujas:
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a GENOTONORM KABIPEN 5,3 mg POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 12 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 0,2 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 0,4 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere receta
Médicos online para GENOTONORM KABIPEN 5,3 mg POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de GENOTONORM KABIPEN 5,3 mg POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes





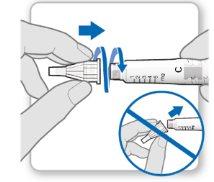
 Uso del cubreagujas (opcional)
Uso del cubreagujas (opcional)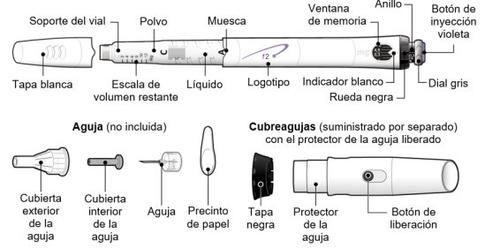
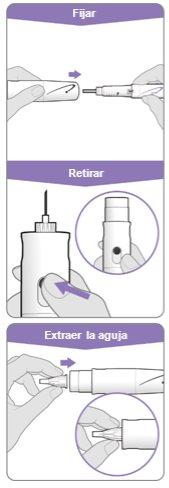 Fijar el cubreagujas:
Fijar el cubreagujas:





