ГИНЕ-КАНЕСТЕН 500 МГ МЯГКИЕ ВАГИНАЛЬНЫЕ КАПСУЛЫ


Инструкция по применению ГИНЕ-КАНЕСТЕН 500 МГ МЯГКИЕ ВАГИНАЛЬНЫЕ КАПСУЛЫ
Введение
Прошпект: информация для пользователя
Гин-Канестен 500 мг вагинальная мягкая капсула
клотримазол
Прочитайте внимательно весь прошпект перед началом использования этого лекарства, поскольку он содержит важную информацию для вас.
Следуйте точно инструкциям по применению лекарства, изложенным в этом прошпекте или указанным вашим врачом или фармацевтом.
- Сохраните этот прошпект, поскольку вам может понадобиться прочитать его снова.
- Если вам нужен совет или дополнительная информация, проконсультируйтесь с вашим фармацевтом.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этом прошпекте. См. раздел 4,
Вы должны проконсультироваться с врачом, если ваше состояние ухудшается или не улучшается после 3 дней.
1. Что такое Гин-Канестен и для чего он используется
Клотримазол - это антимикотическое средство (лекарство, используемое для лечения инфекций, вызванных грибами).
Это лекарство показано для лечения неосложненной вульвовагинальной кандидозной инфекции (вагинальной инфекции, вызванной грибом под названием Candida) (см. раздел «Предостережения и меры предосторожности»).
Основные симптомы включают зуд, обычно сопровождаемый увеличением вагинальных выделений, боль и покраснение вагинальной и вульварной зоны, жжение и ощущение жара при мочеиспускании. Эти симптомы не являются специфическими для вульвовагинальной кандидозной инфекции. В случае сомнений обратитесь к вашему врачу.
2. Что вам нужно знать перед началом использования Гин-Канестена
Не используйте Гин-Канестен
- Если вы аллергичны (гиперчувствительны) к клотримазолу, имидазолам в целом или к любому другому компоненту этого лекарства (включая раздел 6).
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед началом использования Гин-Канестена.
Перед использованием этого лекарства сообщите вашему врачу, если у вас есть проблемы с иммунной системой, например, если вы проходите лечение с помощью пероральных кортикостероидов или если у вас есть ВИЧ-инфекция, СПИД или диабет.
Вы должны проконсультироваться с врачом, если симптомы ухудшаются во время лечения или сохраняются после 3 дней или если наблюдается увеличение вагинальных выделений или изменения их вида или запаха, или если у вас出现ило вагинальное кровотечение.
В случае лихорадки (38°C или выше), боли в животе или спине, боли в нижней части спины, обильных водянистых вагинальных выделений и/или тошноты, вам следует проконсультироваться с вашим врачом, чтобы исключить другие виды заболеваний.
В случае возникновения аллергической реакции во время использования вам следует прекратить лечение и немедленно обратиться к вашему врачу. Признаки тяжелой аллергической реакции включают приподнятую и зудящую сыпь, отек, иногда на лице или во рту, что может вызвать затруднение дыхания.
Не следует использовать тампоны, вагинальные душевые, спермициды или другие вагинальные продукты во время использования этого лекарства, для получения дополнительной информации см. раздел «Использование Гин-Канестена с другими лекарствами».
Не рекомендуется начинать лечение во время менструации. Лечение должно быть завершено до начала менструации.
Это лекарство может уменьшить эффективность и безопасность латексных изделий, таких как презервативы и диафрагмы. Этот эффект является временным и возникает только во время лечения.
Рекомендуется избегать половых контактов в случае вагинальной инфекции и во время использования этого лекарства, чтобы предотвратить заражение партнера.
Дети
Не использовать у детей младше 12 лет.
Использование Гин-Канестена с другими лекарствами
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принимать любое другое лекарство, особенно если вы принимаете такролимус или сиролимус (лекарства, используемые у пациентов после трансплантации).
Беременность и лактация
Если вы беременны или в период лактации, или если вы считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства.
Беременность
Клотримазол может быть использован во время беременности, но только под наблюдением медицинского специалиста.
В случае лечения этим лекарством в последние 4-6 недель беременности рекомендуется воздержаться от использования аппликатора, вводя вагинальную капсулу непосредственно пальцем, предварительно тщательно вымыв руки.
Лактация
Клотримазол может быть использован во время лактации.
Вождение и использование машин
Влияние Гин-Канестена на способность управлять транспортными средствами и работать с машинами является незначительным или отсутствует.
3. Как использовать Гин-Канестен
Следуйте точно инструкциям по применению этого лекарства, изложенным в этом прошпекте или указанным вашим врачом или фармацевтом. В случае сомнений обратитесь к вашему врачу или фармацевту.
Это лекарство вводится вагинально. Не принимайте внутрь.
Рекомендуемая доза:
Взрослые и подростки старше 12 лет
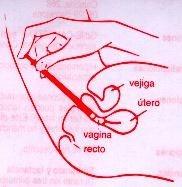
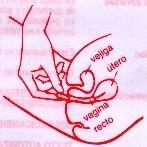
Обычно достаточно одной вагинальной капсулы, вводимой предпочтительно вечером перед сном. Вагинальная капсула должна быть введена глубоко в вагину (см. инструкции по использованию аппликатора), пациентка должна лежать на спине с немного согнутыми ногами.
Если симптомы сохраняются после окончания лечения или повторяются через два месяца, или если у вас есть проблемы с иммунной системой, ВИЧ-инфекция, СПИД или диабет, вам следует проконсультироваться с врачом.
Беременные женщины
В случае лечения в последние 4-6 недель беременности рекомендуется не использовать аппликатор, вводя вагинальную капсулу непосредственно пальцем, предварительно тщательно вымыв руки.
ИНСТРУКЦИИ ПО ПРИМЕНЕНИЮ
ИНСТРУКЦИИ ПО ИСПОЛЬЗОВАНИЮ АППЛИКАТОРА
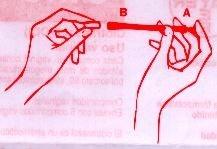
Поместите вагинальную капсулу внутрь аппликатора Б.
|
|
ВВЕДЕНИЕ ВАГИНАЛЬНОЙ КАПСУЛЫ БЕЗ АППЛИКАТОРА Предостережение Беременные женщины должны строго следовать инструкциям по применению, указанным их врачом. |
|
Если вы примете больше Гин-Канестена, чем следует
Случайное проглатывание может вызвать желудочно-кишечные расстройства и/или рвоту. Случайное введение в глаза может вызвать жжение и раздражение глаз без серьезных последствий.
В случае передозировки или случайного приема проконсультируйтесь немедленно с вашим врачом или фармацевтом или позвоните в Службу токсикологической информации. Телефон (91) 562 04 20, указав лекарство и количество, принятое внутрь.
4. Возможные побочные эффекты
Как и все лекарства, Гин-Канестен может вызывать побочные эффекты, хотя не все люди испытывают их.
Побочные реакции с неизвестной частотой (не могут быть оценены на основе доступных данных) включают:
Расстройства иммунной системы:
Ангиоэдема (отек под кожей), аллергическая реакция, гиперчувствительность.
Расстройства сосудов:
Обморок (внезапная потеря сознания, обморок), гипотония (пониженное артериальное давление).
Расстройства дыхательной системы, грудной клетки и средостения:
Затруднение дыхания.
Расстройства желудочно-кишечного тракта:
Боль в животе, тошнота.
Расстройства кожи и подлежащих тканей:
Сыпь, уртикария (поднятая, красная, зудящая сыпь).
Расстройства репродуктивной системы и молочной железы:
Вагинальная десквамация, вагинальные выделения, вульвовагинальный зуд, вульвовагинальная эритема, ощущение жара в генитальной зоне, вульвовагинальные расстройства, вульвовагинальная боль и вагинальное кровотечение.
Общие расстройства и местные реакции:
Раздражение места применения, отек, боль.
Эти симптомы обычно не требуют прекращения лечения и чаще всего возникают в первые дни лечения.
Сообщение о побочных эффектах:
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не указаны в этом прошпекте. Вы также можете сообщить о них напрямую через Систему фармакологического надзора за лекарствами для человека: http;//www.notificaram.es. Сообщая о побочных эффектах, вы можете способствовать предоставлению более полной информации о безопасности этого лекарства
5. Хранение Гин-Канестена
Храните в оригинальной упаковке для защиты от влаги
Держите это лекарство вне поля зрения и досягаемости детей.
Не используйте Гин-Канестен после истечения срока годности, указанного на упаковке, после аббревиатуры СРОК. Срок годности - последний день месяца, указанного.
Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в специальный пункт приема в аптеке. В случае сомнений проконсультируйтесь с вашим фармацевтом, как правильно утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы будете способствовать защите окружающей среды.
6. Состав упаковки и дополнительная информация
Состав Гин-Канестена
- Активное вещество - клотримазол. Каждая вагинальная капсула содержит 500 мг клотримазола.
- Другие компоненты (вспомогательные вещества) - мягкая белая вазелиновая мазь, жидкая вазелиновая мазь, желатина, глицерол (Е-422), очищенная вода, диоксид титана (Е-171), желтый хинолин (Е-104), желто-оранжевый С (Е-110), лецитин, среднецепочечные триглицериды
Внешний вид продукта и состав упаковки:
Это лекарство представляет собой вагинальную капсулу желтого цвета с каплевидной формой. Оно выпускается в упаковках, содержащих 1 вагинальную капсулу и вагинальный аппликатор.
Владелец разрешения на маркетинг
БАЙЕР ИСПАНИЯ, С.Л.
Ав. Байкс Льобрегат, 3 – 5
08970 Сант Хоан Деспи (Барселона)
Испания
Производитель
GP ГРЕНЦАХ ПРОДУКЦИОНС ГМБХ
Эмиль-Баррел-Штрассе 7
79639 ГРЕНЦАХ-ВАЙЛЕН
ГЕРМАНИЯДатапоследнегопересмотраэтогопрошпекта:Март 2022
Подробная и актуальная информация о этом лекарстве доступна на сайте Агентства по лекарствам и медицинским изделиям Испании (АЕМПС) http://www.aemps.gob.es/
- Страна регистрации
- Активное вещество
- Требуется рецептНет
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ГИНЕ-КАНЕСТЕН 500 МГ МЯГКИЕ ВАГИНАЛЬНЫЕ КАПСУЛЫФорма выпуска: ВАГИНАЛЬНЫЕ СУППОЗИТОРИИ/КАПСУЛЫ/ТАБЛЕТКИ, 100 мг клотримазолАктивное вещество: КлотримазолПроизводитель: Bayer Hispania S.L.Требуется рецептФорма выпуска: ВАГИНАЛЬНЫЙ ПОЛУЖИДКИЙ ПРЕПАРАТ, 2% клотримазол / 100 гАктивное вещество: КлотримазолПроизводитель: Bayer Hispania S.L.Требуется рецептФорма выпуска: ВАГИНАЛЬНЫЕ СУППОЗИТОРИИ/КАПСУЛЫ/ТАБЛЕТКИ, 500 мг клотримазолАктивное вещество: КлотримазолПроизводитель: Bayer Hispania S.L.Требуется рецепт
Аналоги ГИНЕ-КАНЕСТЕН 500 МГ МЯГКИЕ ВАГИНАЛЬНЫЕ КАПСУЛЫ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ГИНЕ-КАНЕСТЕН 500 МГ МЯГКИЕ ВАГИНАЛЬНЫЕ КАПСУЛЫ в Польша
Аналог ГИНЕ-КАНЕСТЕН 500 МГ МЯГКИЕ ВАГИНАЛЬНЫЕ КАПСУЛЫ в Украина
Врачи онлайн по ГИНЕ-КАНЕСТЕН 500 МГ МЯГКИЕ ВАГИНАЛЬНЫЕ КАПСУЛЫ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ГИНЕ-КАНЕСТЕН 500 МГ МЯГКИЕ ВАГИНАЛЬНЫЕ КАПСУЛЫ – по решению врача и с учетом местных правил.