CLOTRIMAZOL GINE-CANESMED 100 mg VAGINAL TABLETS

How to use CLOTRIMAZOL GINE-CANESMED 100 mg VAGINAL TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information Leaflet
Clotrimazole Gine-Canesmed 100 mg vaginal tablets EFG
clotrimazole
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet:
- What Clotrimazole Gine-Canesmed is and what it is used for
- What you need to know before using Clotrimazole Gine-Canesmed
- How to use Clotrimazole Gine-Canesmed
- Possible side effects
- Storage of Clotrimazole Gine-Canesmed
- Package contents and additional information
1. What Clotrimazole Gine-Canesmed is and what it is used for
Clotrimazole is an antifungal (a medication used to treat fungal infections).
This medication is indicated, in adults and adolescents over 12 years, for the treatment of:
- Complicated vulvovaginal candidiasis (4 or more fungal infections in the last year or infections with severe symptoms or patients with a weakened immune system) and,
- Uncomplicated vulvovaginal candidiasis (sporadic fungal infections or less than 4 infections in the last year or infections with mild to moderate symptoms).
2. What you need to know before using Clotrimazole Gine-Canesmed
Do not use Clotrimazole Gine-Canesmed
If you are allergic to clotrimazole, imidazoles in general, or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to use Clotrimazole Gine-Canesmed.
Before using this medication, inform your doctor if you have problems with your immune system, such as if you are being treated with oral corticosteroids or have HIV, AIDS, or diabetes.
In case of fever (38°C or higher), abdominal or back pain, lumbar pain, abundant watery vaginal discharge, and/or nausea, you should consult your doctor to rule out other types of illness.
You should not use tampons, vaginal douches, spermicides, or other vaginal products while using this medication.
It is not recommended to start treatment during menstruation. Treatment should have ended before the start of menstruation.
This medication may reduce the effectiveness and safety of latex products, such as condoms and diaphragms. This effect is temporary and only occurs during treatment.
It is recommended to avoid sexual intercourse in case of vaginal infection and while using this medication to prevent the partner from becoming infected.
In case of an allergic reaction during use, treatment should be discontinued and your doctor consulted immediately. Signs of a severe allergic reaction include a swollen and itchy rash, swelling, sometimes on the face or mouth, which can cause difficulty breathing.
You should consult a doctor if symptoms worsen during treatment or persist after completion of the treatment, or if there is an increase in vaginal discharge or changes in its appearance or odor, or if you experience vaginal bleeding.
Children and adolescents
This medication is indicated in children over 12 years, see below How to use Clotrimazole Gine-Canesmedfor administration instructions.
Using Clotrimazole Gine-Canesmed with other medications
Inform your doctor or pharmacist that you are taking, have recently taken, or may need to take any other medication, especially if you are taking tacrolimus or sirolimus (medications used in transplant patients), so that your doctor can monitor you properly.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy
Clotrimazole may be used during pregnancy, but only under the supervision of a healthcare professional.
Breastfeeding
Clotrimazole may be used during breastfeeding.
Driving and using machines
The influence of Clotrimazole Gine-Canesmed on the ability to drive and use machines is negligible or non-existent.
3. How to use Clotrimazole Gine-Canesmed
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Vaginal administration. Do not swallow.
The recommended dose is:
Adults and adolescents over 12 years
Complicated vulvovaginal candidiasis (4 or more fungal infections in the last year or infections with severe symptoms or patients with a weakened immune system): Apply one vaginal tablet preferably at night for 12 consecutive days.
Uncomplicated vulvovaginal candidiasis (sporadic fungal infections or less than 4 infections in the last year or infections with mild to moderate symptoms): Apply one vaginal tablet preferably at night for 6 consecutive days.
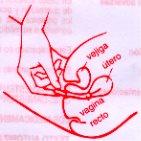
The tablet should be inserted deeply into the vagina with the finger (see below), with the patient lying on their back and with their legs slightly bent.
INSERTION OF THE VAGINAL TABLET |
|
The vaginal tablets require adequate moisture conditions in the vagina for optimal dissolution of the medication. If these conditions are not met, part of the medication may not dissolve completely and may come out of the vagina. To prevent this, it is essential to insert the tablet as deeply as possible into the vagina at bedtime. If the vaginal tablet does not dissolve completely after the first administration, consult your doctor or pharmacist to assess the use of alternative treatments.
If symptoms persist after completing the treatment or recur after two months, or if you have problems with your immune system, HIV, AIDS, or diabetes, you should consult your doctor.
Eye contact should be avoided, as it may cause irritation. If accidental eye contact occurs, rinse with plenty of water and consult an ophthalmologist if necessary.
If you use more Clotrimazole Gine-Canesmed than you should
Accidental ingestion may cause gastrointestinal discomfort and/or vomiting.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service. Phone (91) 562 04 20, indicating the medication and the amount ingested.
If you forget to use Clotrimazole Gine-Canesmed
In case of a missed dose, wait for the next one. Do not apply a double dose to make up for the missed dose.
If you interrupt treatment with Clotrimazole Gine-Canesmed
Continue using Clotrimazole Gine-Canesmed until the treatment is completed, even if you feel better. You need to complete the full treatment to cure the infection. If you interrupt the treatment, the fungus may not have disappeared.
If you have any other questions about using this medication, ask your doctor.
4. Possible side effects
Like all medications, Clotrimazole Gine-Canesmed can cause side effects, although not everyone experiences them.
Adverse reactions with unknown frequency (cannot be estimated from available data) are:
Angioedema (swelling under the skin), allergic reaction, hypersensitivity.
Syncope (sudden loss of consciousness, fainting), hypotension (low blood pressure).
Difficulty breathing.
Abdominal pain, nausea.
Rash, urticaria (elevated red itchy patches).
Vaginal scaling, vaginal discharge, vulvovaginal itching, vulvovaginal erythema, burning sensation in the genital area, vulvovaginal discomfort, vulvovaginal pain, and vaginal bleeding.
Irritation at the application site, edema, pain.
These symptoms usually do not require discontinuation of treatment and are more common during the first days of treatment.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: http://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Clotrimazole Gine-Canesmed
Keep this medication out of the sight and reach of children.
Store at a temperature below 25°C. Store in the original packaging.
Do not use Clotrimazole Gine-Canesmed after the expiration date stated on the packaging, after the abbreviation CAD. The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and any unused medication in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Composition of Clotrimazole Gine-Canesmed
- The active ingredient is clotrimazole. Each vaginal tablet contains 100 mg of clotrimazole.
- The other components (excipients) are: lactose monohydrate, microcrystalline cellulose (E460i), lactic acid (E270), calcium lactate pentahydrate, cornstarch, hypromellose, colloidal anhydrous silica, crospovidone (E1202), and magnesium stearate.
Appearance of the product and package contents
White, oblong vaginal tablets.
They are presented in a cardboard box containing a blister pack (polyamide/aluminum foil/polyvinyl chloride heat-sealed to a hard aluminum foil) with 6 vaginal tablets.
Marketing authorization holder and manufacturer
Marketing authorization holder
BAYER HISPANIA, S.L.
Av. Baix Llobregat, 3 – 5
08970 Sant Joan Despí (Barcelona)
Spain
Manufacturer
GP Grenzach Produktions GmbH
Emil-Barell-Strasse 7
79639 Grenzach-Wyhlen
Germany
Date of the last revision of this leaflet: February 2022
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price3.47 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CLOTRIMAZOL GINE-CANESMED 100 mg VAGINAL TABLETSDosage form: VAGINAL SEMISOLID, 2% clotrimazole / 100 gActive substance: clotrimazoleManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 500 mg clotrimazoleActive substance: clotrimazoleManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 100 mg clotrimazoleActive substance: clotrimazoleManufacturer: Bayer Hispania S.L.Prescription not required
Online doctors for CLOTRIMAZOL GINE-CANESMED 100 mg VAGINAL TABLETS
Discuss questions about CLOTRIMAZOL GINE-CANESMED 100 mg VAGINAL TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions