ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ
Спросите врача о рецепте на ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ

Инструкция по применению ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ
Введение
Инструкция:информация для пользователя
Фулвестрант Тева 250 мг раствор для инъекций в предварительно заполненном шприце EFG
Прочитайте внимательно всю инструкцию перед началом использования этого лекарства,поскольку она содержит важную информацию для вас.
- Сохраните эту инструкцию, поскольку вам может потребоваться перечитать ее.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой.
- Это лекарство было назначено только вам, и его не следует давать другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
Содержание инструкции
- Что такое Фулвестрант Тева и для чего он используется
- Что вам нужно знать перед началом использования Фулвестранта Тева
- Как использовать Фулвестрант Тева
- Возможные побочные эффекты
- Хранение Фулвестранта Тева
- Содержание упаковки и дополнительная информация
1. Что такое Фулвестрант Тева и для чего он используется
Фулвестрант Тева содержит активное вещество фулвестрант, которое относится к группе блокаторов эстрогена. Эстрогены, тип женских половых гормонов, могут быть в некоторых случаях вовлечены в развитие рака молочной железы.
Фулвестрант Тева используется:
- один, для лечения женщин в постменопаузе с типом рака молочной железы, называемым раком молочной железы с положительным рецептором эстрогена, который является местно расширенным или распространившимся на другие части тела (метастатическим) или,
- в комбинации с палбоцииблибом для лечения женщин с типом рака молочной железы, называемым раком молочной железы с положительным гормональным рецептором, раком молочной железы с отрицательным рецептором 2 фактора роста эпидермиса человека, который является местно расширенным или распространившимся на другие части тела (метастатическим). Женщины, которые не достигли менопаузы, также будут лечены лекарством, называемым агонистом гормона, выделяющего гонадотропин (ГнРГ).
Фулвестрант может быть введен в комбинации с палбоцииблибом. Важно, чтобы вы также прочитали инструкцию к палбоцииблибу. Если у вас есть какие-либо вопросы о палбоцииблибе, проконсультируйтесь с вашим врачом.
2. Что вам нужно знать перед началом использования Фулвестранта Тева
Не используйтеФулвестрант Тева:
- если вы аллергичны к фулвестранту или любому другому компоненту этого лекарства (указанному в разделе 6)
- если вы беременны или в период лактации (см. раздел «Беременность и лактация»)
- если у вас есть тяжелые проблемы с печенью
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом, фармацевтом или медсестрой перед началом использования Фулвестранта Тева, если что-либо из этого относится к вам:
- проблемы с почками или печенью
- низкий уровень тромбоцитов (которые помогают свертыванию крови) или кровотечения
- предыдущие проблемы с тромбозом
- остеопороз (потеря плотности костей)
- алкоголизм (см. раздел «Фулвестрант Тева содержит этанол 96% (алкоголь)»).
Не было изучено эффективность и безопасность фулвестранта (как монотерапии или в комбинации с палбоцииблибом) у пациентов с критической визцеральной болезнью.
Дети и подростки
Фулвестрант Тева не показандетям и подросткам младше 18 лет.
Использование Фулвестранта Тевасдругими лекарствами
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принимать любое другое лекарство.
В частности, вы должны сказать вашему врачу, если вы используете антикоагулянты (лекарства для предотвращения тромбозов).
Беременность илактация
Не используйтеФулвестрант Тева, если вы беременны. Если вы можете забеременеть, вы должны использовать эффективный метод контрацепции во время лечения Фулвестрантом Тева и в течение 2 лет после последней дозы.
Не кормите грудьюво время лечения Фулвестрантом Тева.
Вождение и использование машин
Не ожидается, что Фулвестрант Тева повлияет на вашу способность управлять транспортными средствами или использовать машины. Однако, если вы чувствуете усталость после лечения, не управляйтетранспортными средствами и не используйтемашины.
Фулвестрант Тевасодержит этанол 96% (алкоголь)
Это лекарство содержит 474 мг этанола (алкоголя) в каждой предварительно заполненной инъекционной системе 5 мл, что соответствует 94,8 мг/мл. Количество в дозе 10 мл этого лекарства эквивалентно менее 24 мл пива или 10 мл вина.
Небольшое количество алкоголя в этом лекарстве не окажет заметного влияния.
Фулвестрант Тевасодержит бензиловый спирт
Это лекарство содержит 500 мг бензилового спирта в каждой предварительно заполненной инъекционной системе 5 мл, что эквивалентно 100 мг/мл.
Бензиловый спирт может вызывать аллергические реакции.
Проконсультируйтесь с вашим врачом или фармацевтом, если у вас есть заболевания печени или почек. Это связано с тем, что могут накапливаться в организме большие количества бензилового спирта и вызывать побочные эффекты (метаболический ацидоз).
Фулвестрант Тевасодержит бензоат бензила
Это лекарство содержит 750 мг бензоата бензила в каждой предварительно заполненной инъекционной системе 5 мл, что эквивалентно 150 мг/мл.
3. Как использовать Фулвестрант Тева
Следуйте точно инструкциям по введению этого лекарства, указанным вашим врачом или фармацевтом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом.
Рекомендуемая доза составляет 500 мг фулвестранта (две инъекции по 250 мг/5 мл) однократно в месяц с дополнительной дозой 500 мг, введенной через 2 недели после начальной дозы.
Ваш врач или медсестра введет Фулвестрант Тева путем медленной внутримышечной инъекции в каждый из ваших ягодиц.
Если у вас есть какие-либо другие вопросы о использовании этого лекарства, спросите вашего врача, фармацевта или медсестру.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Вам может потребоваться срочная медицинская помощь, если вы испытываете любой из следующих побочных эффектов:
- аллергические реакции (гиперчувствительность), включая отек лица, губ, языка и/или горла, которые могут быть признаками анафилактических реакций
- тромбоэмболия (повышенный риск тромбозов)*
- воспаление печени (гепатит)
- печеночная недостаточность.
Сообщите вашему врачу, фармацевту или медсестре, если вы заметите любой из следующих побочных эффектов:
Побочные эффекты, зарегистрированные у пациентов, леченных фулвестрантом в монотерапии:
Очень часто:могут встречаться более чем у 1 из 10 человек
- реакции в месте инъекции, такие как боль и/или воспаление
- анормальные уровни печеночных ферментов (в анализе крови)*
- тошнота (чувство дискомфорта)
- слабость, усталость*
- суставная и мышечная боль
- приливы
- кожная сыпь
- аллергические реакции (гиперчувствительность), включая отек лица, губ, языка и/или горла
Все остальные побочные эффекты:
Часто:могут встречаться до 1 из 10 человек
- головная боль
- рвота, диарея или потеря аппетита*
- инфекции мочевыводящих путей
- боль в спине*
- повышение уровня билирубина (пигмента желчи, производимого печенью).
- тромбоэмболия (повышенный риск тромбозов)*
- пониженный уровень тромбоцитов (тромбоцитопения)
- вагинальное кровотечение
- боль в пояснице, отражающаяся на одной стороне ноги (ишиас)
- внезапная слабость, онемение, покалывание или потеря движения в ноге, особенно на одной стороне тела, внезапные проблемы с ходьбой или равновесием (периферическая невропатия)
Редко:могут встречаться до 1 из 100 человек
- густой, белый вагинальный секрет и кандидоз (инфекция)
- гематома и кровотечение в месте инъекции
- повышение уровня гамма-ГТ, печеночного фермента, определяемого в анализе крови
- воспаление печени (гепатит)
- печеночная недостаточность
- онемение, покалывание и боль
- анафилактические реакции.
*Включает побочные эффекты, для которых нельзя оценить точную роль Фулвестранта Тева из-за основного заболевания.
Побочные эффекты, зарегистрированные у пациентов, леченных фулвестрантом в комбинации с палбоцииблибом:
Очень часто:могут встречаться более чем у 1 из 10 человек
- понижение уровня нейтрофилов (нейтропения)
- понижение уровня лейкоцитов (лейкопения)
- инфекции
- усталость
- тошнота
- понижение уровня красных кровяных телец (анемия)
- воспаление или язвы во рту
- диарея
- понижение уровня тромбоцитов (тромбоцитопения)
- рвота
- потеря волос
- кожная сыпь
- потеря аппетита
- лихорадка
Часто:могут встречаться до 1 из 10 человек
- чувство слабости
- повышение уровня печеночных ферментов
- потеря вкуса
- носовое кровотечение
- слизь в глазах
- сухость кожи
- размытое зрение
- сухость глаз
Редко:могут встречаться до 1 из 100 человек
- лихорадка с другими признаками инфекции (фебрильная нейтропения).
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это возможные побочные эффекты, которые не указаны в этой инструкции. Вы также можете сообщить об этом напрямую через систему фармаковигиланса лекарственных средств для человека: https://www.notificaram.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение Фулвестранта Тева
Хранитеэто лекарствов недоступном для детей месте.
Не используйте это лекарство после истечения срока годности, указанного на упаковке или на этикетках шприцев после «CAD». Срок годности - последний день месяца, указанного.
Не используйте это лекарство, если вы заметите частицы или изменение цвета перед введением.
Храните и перевозите в холодильнике (при температуре между 2°C и 8°C).
Отклонения температуры от диапазона между 2°C и 8°C должны быть контролируемы. Это включает в себя избежание хранения при температурах выше 25°C и то, что это не превышает период 4 месяцев, в течение которого средняя температура хранения лекарства ниже 25°C (но выше между 2°C и 8°C). После отклонений температуры лекарство должно быть немедленно возвращено к рекомендуемым условиям хранения (хранить и перевозить в холодильнике при температуре между 2°C и 8°C). Отклонения температуры имеют накопительный эффект на качество лекарства, не должны превышать период 4 месяцев сверх срока годности 2 лет Фулвестранта Тева. Воздействие температур ниже 2°C не повредит лекарство, если только оно не хранится ниже -20°C.
Храните предварительно заполненный шприц в оригинальной упаковке, чтобы защитить его от света.
Ваш медицинский работник будет отвечать за правильное хранение, использование и утилизацию Фулвестранта Тева.
Это лекарство может представлять опасность для водной среды. Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в пункт сбора SIGRE в аптеке. Если у вас есть сомнения, спросите вашего фармацевта, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Фулвестранта Тева
- Активное вещество - фулвестрант. Каждая предзагруженная шприц содержит 250 мг фулвестранта. Каждый мл раствора содержит 50 мг фулвестранта.
- Другие компоненты (вспомогательные вещества) - этанол (96%), бензиловый спирт, бензоат бензила и рафинированное касторовое масло.
Внешний вид продукта и содержание упаковки
Фулвестрант Тева - это вязкая, прозрачная, бесцветная или желтоватая жидкость в предзагруженной шприце, оснащенной соединителем Luer-Lock, содержащей 5 мл инъекционного раствора. Для получения рекомендуемой месячной дозы 500 мг необходимо ввести две шприцы.
Фулвестрант Тева выпускается в двух формах:
- 1 упаковка, содержащая 1 стеклянную предзагруженную шприцу и 1 иглу с системой безопасности для соединения с корпусом шприцы.
- 1 упаковка, содержащая 2 стеклянные предзагруженные шприцы, и предоставляются 2 иглы с системой безопасности для соединения с корпусом шприцы.
Возможно, что будут продаваться только некоторые размеры упаковок.
Владелец разрешения на маркетинг и производитель
Владелец разрешения на маркетинг
Teva Pharma, S.L.U.
C/ Anabel Segura, 11. Edificio Albatros B, 1-я пл.
28108 Alcobendas, Madrid
Испания
Производитель
Pliva Croatia Ltd.
Prilaz baruna Filipovica 25
10000 Zagreb
Хорватия
Это лекарство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Австрия: Фулвестрант ратиофарм 250 мг инъекционный раствор в предзагруженной шприце
Бельгия: Фулвестрант Тева 250 мг раствор для инъекции/раствор для инъекции/инъекционный раствор
Болгария: ??????????? ???? 250 мг ?????????? ??????? ? π???????????? ??π?????? ?π????????
Хорватия: Фулвестрант Плива 250 мг оtopина за инъекцию в наполненной шприце
Чехия: Фулвестрант Тева
Дания: Фулвестрант Тева
Финляндия: Фулвестрант ратиофарм 250 мг инъекционный раствор, жидкость, предзагруженная шприца
Франция: Фулвестрант Тева 250мг раствор для инъекции в предзагруженной шприце
Германия: Фулвестрант Тева 250 мг инъекционный раствор в предзагруженной шприце
Венгрия: Фулвестрант Тева 250 мг/5 мл раствор для инъекции в предзагруженной шприце
Исландия: Фулвестрант Тева 250 мг стунгульф, раствор в предзагруженной шприце
Ирландия: Фулвестрант Тева 250 мг раствор для инъекции в предзагруженной шприце
Италия: Фулвестрант Тева
Латвия: Фулвестрант Тева 250 мг шкидумс для инъекций в предзагруженной шприце
Литва: Фулвестрант Тева 250мг инъекционный раствор в предзагруженной шприце
Люксембург: Фулвестрант Тева 250 мг раствор для инъекции/раствор для инъекции/инъекционный раствор
Нидерланды: Фулвестрант Тева 250 мг, раствор для инъекции в предзагруженной шприце
Польша: Фулвестрант Тева
Португалия: Фулвестрант Тева
Румыния: Фулвестрант Тева 250 мг раствор для инъекции в предзагруженной шприце
Словакия: Фулвестрант Тева 250 мг
Словения: Фулвестрант Тева 250 мг разtopина для инъекций в наполненной инъекционной шприце
Испания: Фулвестрант Тева 250 мг раствор для инъекции в предзагруженной шприце ЕФГ
Швеция: Фулвестрант Тева 250 мг инъекционный раствор, жидкость, предзагруженная шприца
Великобритания: Фулвестрант Тева 250мг раствор для инъекции в предзагруженной шприце
(Северная Ирландия)
Дата последнего пересмотра этой инструкции: Июль 2025
Другие источники информации
Подробная информация о этом лекарстве доступна на сайте Агентства по лекарственным средствам и медицинским изделиям Испании (АЕМПС) https://www.aemps.gob.es/
Вы можете получить доступ к подробной и актуальной информации о этом лекарстве, отсканировав код QR, включенный в упаковку, с помощью своего мобильного телефона (смартфона). Вы также можете получить доступ к этой информации на следующем сайте: https://cima.aemps.es/cima/dochtml/p/80910/P_80910.html
Код QR + URL
----------------------------------------------------------------------------------------------------------------------------------
Эта информация предназначена только для медицинских специалистов:
Фулвестрант Тева 500 мг (2 х 250 мг/5 мл инъекционный раствор) должен быть введен с помощью двух предзагруженных шприц (см. раздел 3).
Инструкции по введению
Ввести инъекцию в соответствии с местными рекомендациями по внутримышечному введению больших объемов.
ПРИМЕЧАНИЕ: Из-за близости седалищного нерва необходимо проявлять осторожность при введении Фулвестранта Тева в дорсоглютейную зону (см. раздел 4.4).
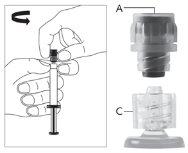
Предупреждение - НЕ стерилизуйте иглу с системой безопасности в автоклаве перед использованием. Руки должны оставаться позади иглы во все время использования и утилизации.
Для каждой из двух шприц:
| Фигура 1
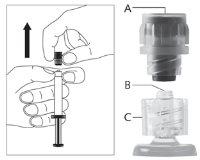
|
| Фигура 2
|
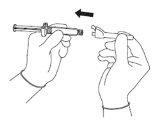
| Фигура 3
|
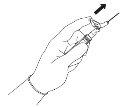
| Фигура 4
|
ПРИМЕЧАНИЕ: Активируйте, находясь на расстоянии от своего тела и других людей. Слушайте щелчок и визуально подтверждайте, что кончик иглы полностью защищен. | Фигура 5
|
Утилизация
Предзагруженные шприцы предназначены толькодля одноразового использования.
Это лекарство может представлять опасность для водной среды. Утилизация неиспользованного лекарства и всех материалов, которые были в контакте с ним, должна осуществляться в соответствии с местными правилами.

Сколько стоит ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в Испании в 2025 году?
Средняя цена на ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в декабрь, 2025 года составляет около 225.7 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках225.7 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 250 мг/5 млАктивное вещество: ФулвестрантПроизводитель: Bexal Farmaceutica S.A.Требуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 250 мгАктивное вещество: ФулвестрантПроизводитель: Ever Valinject GmbhТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 250 мгАктивное вещество: ФулвестрантПроизводитель: Astrazeneca AbТребуется рецепт
Аналоги ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в Польща
Аналог ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в Україна
Врачи онлайн по ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ФУЛВЕСТРАНТ ТЕВА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ – по решению врача и с учетом местных правил.